Here are the essential concepts you must grasp in order to answer the question correctly.
Ideal Gas Law
The Ideal Gas Law relates the pressure, volume, temperature, and number of moles of an ideal gas through the equation PV = nRT. This law is fundamental in understanding gas behavior under various conditions and is essential for calculating changes in state variables during processes like compression.
Recommended video:
Ideal Gases and the Ideal Gas Law
Work Done on a Gas
In thermodynamics, the work done on a gas during a volume change is calculated using the formula W = -∫PdV, where P is the pressure and dV is the change in volume. This concept is crucial for determining how much energy is transferred to or from the gas during processes such as compression or expansion.
Recommended video:
Calculating Work Done on Monoatomic Gas
Constant c in the Process Equation
In the given process equation p = cV^2, the constant c represents a proportionality factor that relates pressure to the square of volume. Understanding how this relationship affects the pressure during the compression process is key to calculating the work done on the gas, as it influences the pressure at different volumes.
Recommended video:
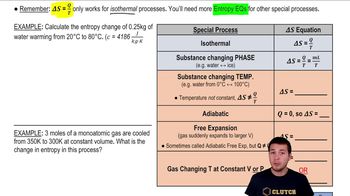
Entropy Equations for Special Thermodynamic Processes
 Knight Calc 5th Edition
Knight Calc 5th Edition Ch 19: Work, Heat, and the First Law of Thermodynamics
Ch 19: Work, Heat, and the First Law of Thermodynamics Problem 19
Problem 19 Verified step by step guidance
Verified step by step guidance