Hey, guys. So a lot of times in our physics equations we usually use the mass of a material. For example, our most important equation, F equals ma, we use the mass to calculate the force. But, a lot of times in thermodynamics, you're going to need to know the number of moles or the number of particles that make up a material like a gas. So, I'm going to introduce to you the moles and also Avogadro's number in this video, and I'm also going to show you a systematic way to convert between these three related ideas: the mass, the mole, and the number of particles. If you've ever taken a chemistry class, you're probably familiar with the mole, but if not, I'm going to go ahead and introduce it to you. Let's check this out. The mole, which is given by the letter little n, is really just the amount of material. It's an amount of stuff that is equal to a very specific number of particles, 6.022×1023. This really big number here is known as Avogadro's number, has a special name, and we also give it the letter big Na, a for Avogadro. So a mole is really just a shortcut way of saying it's an amount that's equal to this many particles. It's kind of like if you said if you had a dozen donuts, that dozen just means you have 12. A mole just means that you have this many of whatever it is that you're talking about, and it actually applies to any substance that you talk about. For example, if you have a mole of nitrogen, that just means 6.022×1023 nitrogen atoms, it's Avogadro's number. If you have a mole of H2O, it just means that you have an Avogadro's number of H2O molecules. If you have a mole of dollars, you'd be an extremely rich person because you would have an Avogadro's number of dollars. That's just a, you know, a mole just means a shortcut way of saying this many particles or whatever it is. Now as we said before, in a lot of our thermodynamics discussions and equations, we're gonna be talking about these sort of 3 related ideas, the mass, the mole, and also the number of particles, and specifically you're going to need to know how to convert between them. So unfortunately, what happens is that a lot of these terms have sort of similar letters. The number of particles is given by big N, the number of moles is given by little n, and then the mass of something is given by little m. They're all sort of like, they are sort of similar. Now fortunately, the equations are very straightforward. So let's check this out. If you wanted to connect to the number of particles to the number of moles, we're just gonna use this equation over here. Little n equals big N, the number of particles divided by Avogadro's number. If you have some amount of particles, you wanna figure out how many moles, you just have to divide it by this really really big number here,6.022×1023, the number of particles, and it'll get you that many moles. So let's go ahead and check out our first example here. So we want to calculate how many moles are in this many carbon atoms. So what are we trying to do? We're trying to go from a number of moles, which is little n, and we're trying to get I'm sorry. We're trying to get go from a number of particles, big N, and we're trying to get to the number of moles. So we're really just trying to go from here to here, and therefore, we're just gonna use this equation. Alright? So let's do it. So we have little n is equal to big N over Avogadro's number. So this is gonna be 8.33×1037. This is gonna be particles, and then we're gonna divide it by Avogadro's number 6.022×1023. The units for this are basically particles per mole. So what you'll see here is the particles and over particles will cancel out and you're just left with moles. Now if you go ahead and plug this into your calculator, you're gonna get 1.38×1014, and this is how many moles of carbon atoms you have. That's the answer. Right? So you just figure out on this flowchart here which two variables you're trying to connect and then use the equation. Let's check out the second example. We're trying to calculate how many grams of aluminum is in this many moles of aluminum. So here we're going from moles and now we want to calculate a gram, which is a mass. So here on our flowchart, we're actually trying to go from here to here, so we're gonna need another equation to do this. And that equation is little m, the mass, divided by big M. So this big M here is called the molar mass of a material. It's also known for those of you who've taken chemistry as the atomic mass, which you can find in any periodic table. So fortunately here at Clutch, we have our own periodic table you can find at the bottom of this page here. And if you go ahead and click on aluminum, what you're going to get is that the atomic mass is 26.98. That's the molar mass. What that means is that there are that many grams per 1 mole of aluminum. All right? Now one thing I want to mention here is that one way to kind of remember these equations is that little n is always equal to the two letters divided. So big N over little, big N over Avogadro's number, there are 2 big N's and also little m over big M. That might be one way that you remember that. Right? So let's go ahead and use this equation here. We have n equals little m divided by big M, but we want to calculate this little m here. That's the mass. So we rearrange this equation and we're going to get m is equal to n times, this is going to be big M. So the number of moles that we have is 2.35 and the molar mass of aluminum we were given is 26.98. So this is 26.98 like this, and if you go ahead and work this out, you're gonna get a mass of 63.4, and that's grams of aluminum. Alright. So that's the answer, that's 63.4 grams. Let's check out the third one. Now we want to calculate how many particles are in 24 grams of H2O. So here what we have is we have a mass and we're actually trying to get actually let me lay it out this way. So we have a mass, and we want to get to the number of particles. Remember, that is big N. So in our diagram, we're actually trying to go from this little m and we're trying to go all the way back to the number of particles. But in order to do that, we're going to actually have to go through moles. Moles are kind of the bridge between these variables. So if you kind of think about this, if I'm trying to get from here to here and I actually have to first go through moles and then convert it to the number of particles. So that's what we're going to do here. So in this first step, we're just gonna use the equation n equals little m over big M. I want to calculate the number of moles, so I'm just gonna go ahead and plug in my mass and divide it by the molar mass. I'm just gonna so this is gonna be a little m and that is gonna be a big M. So this is my mass which is 24 grams, and this is my molar mass which I'm given is 18.02. So this is 18.02 like this. And if you go ahead and work this out, what you're going to get is 1.33 moles. That's how many that's so many moles of H2O that I have. The second step here is now I actually want to go from moles to number of particles, and I'm actually gonna use the other equation. I'm gonna use my, my n over na equation. So this is, n equals and I have n over na. Now what I'm actually trying to do is calculate n. So I'm just gonna rearrange this equation. This is gonna be n is equal to little n times Avogadro's number. So I have little n, that's the number of moles. Right? It's 1.33 times, and then I've got, 6.022×1023. That's the number of particles. Let me go ahead and move, and I've got the number of particles is equal to 8.01×1023, and that's gonna be particles. Right? That's how many molecules or particles, if you will, of H2O that you will have. Alright? So that's the answer. That's how you sort of navigate this flowchart here. You just figure out where you're trying to go, what are your given and target variables, and use the right equation. Let me know if you guys have any questions.
- 0. Math Review31m
- 1. Intro to Physics Units1h 23m
- 2. 1D Motion / Kinematics3h 56m
- Vectors, Scalars, & Displacement13m
- Average Velocity32m
- Intro to Acceleration7m
- Position-Time Graphs & Velocity26m
- Conceptual Problems with Position-Time Graphs22m
- Velocity-Time Graphs & Acceleration5m
- Calculating Displacement from Velocity-Time Graphs15m
- Conceptual Problems with Velocity-Time Graphs10m
- Calculating Change in Velocity from Acceleration-Time Graphs10m
- Graphing Position, Velocity, and Acceleration Graphs11m
- Kinematics Equations37m
- Vertical Motion and Free Fall19m
- Catch/Overtake Problems23m
- 3. Vectors2h 43m
- Review of Vectors vs. Scalars1m
- Introduction to Vectors7m
- Adding Vectors Graphically22m
- Vector Composition & Decomposition11m
- Adding Vectors by Components13m
- Trig Review24m
- Unit Vectors15m
- Introduction to Dot Product (Scalar Product)12m
- Calculating Dot Product Using Components12m
- Intro to Cross Product (Vector Product)23m
- Calculating Cross Product Using Components17m
- 4. 2D Kinematics1h 42m
- 5. Projectile Motion3h 6m
- 6. Intro to Forces (Dynamics)3h 22m
- 7. Friction, Inclines, Systems2h 44m
- 8. Centripetal Forces & Gravitation7h 26m
- Uniform Circular Motion7m
- Period and Frequency in Uniform Circular Motion20m
- Centripetal Forces15m
- Vertical Centripetal Forces10m
- Flat Curves9m
- Banked Curves10m
- Newton's Law of Gravity30m
- Gravitational Forces in 2D25m
- Acceleration Due to Gravity13m
- Satellite Motion: Intro5m
- Satellite Motion: Speed & Period35m
- Geosynchronous Orbits15m
- Overview of Kepler's Laws5m
- Kepler's First Law11m
- Kepler's Third Law16m
- Kepler's Third Law for Elliptical Orbits15m
- Gravitational Potential Energy21m
- Gravitational Potential Energy for Systems of Masses17m
- Escape Velocity21m
- Energy of Circular Orbits23m
- Energy of Elliptical Orbits36m
- Black Holes16m
- Gravitational Force Inside the Earth13m
- Mass Distribution with Calculus45m
- 9. Work & Energy1h 59m
- 10. Conservation of Energy2h 51m
- Intro to Energy Types3m
- Gravitational Potential Energy10m
- Intro to Conservation of Energy29m
- Energy with Non-Conservative Forces20m
- Springs & Elastic Potential Energy19m
- Solving Projectile Motion Using Energy13m
- Motion Along Curved Paths4m
- Rollercoaster Problems13m
- Pendulum Problems13m
- Energy in Connected Objects (Systems)24m
- Force & Potential Energy18m
- 11. Momentum & Impulse3h 40m
- Intro to Momentum11m
- Intro to Impulse14m
- Impulse with Variable Forces12m
- Intro to Conservation of Momentum17m
- Push-Away Problems19m
- Types of Collisions4m
- Completely Inelastic Collisions28m
- Adding Mass to a Moving System8m
- Collisions & Motion (Momentum & Energy)26m
- Ballistic Pendulum14m
- Collisions with Springs13m
- Elastic Collisions24m
- How to Identify the Type of Collision9m
- Intro to Center of Mass15m
- 12. Rotational Kinematics2h 59m
- 13. Rotational Inertia & Energy7h 4m
- More Conservation of Energy Problems54m
- Conservation of Energy in Rolling Motion45m
- Parallel Axis Theorem13m
- Intro to Moment of Inertia28m
- Moment of Inertia via Integration18m
- Moment of Inertia of Systems23m
- Moment of Inertia & Mass Distribution10m
- Intro to Rotational Kinetic Energy16m
- Energy of Rolling Motion18m
- Types of Motion & Energy24m
- Conservation of Energy with Rotation35m
- Torque with Kinematic Equations56m
- Rotational Dynamics with Two Motions50m
- Rotational Dynamics of Rolling Motion27m
- 14. Torque & Rotational Dynamics2h 5m
- 15. Rotational Equilibrium3h 39m
- 16. Angular Momentum3h 6m
- Opening/Closing Arms on Rotating Stool18m
- Conservation of Angular Momentum46m
- Angular Momentum & Newton's Second Law10m
- Intro to Angular Collisions15m
- Jumping Into/Out of Moving Disc23m
- Spinning on String of Variable Length20m
- Angular Collisions with Linear Motion8m
- Intro to Angular Momentum15m
- Angular Momentum of a Point Mass21m
- Angular Momentum of Objects in Linear Motion7m
- 17. Periodic Motion2h 9m
- 18. Waves & Sound3h 40m
- Intro to Waves11m
- Velocity of Transverse Waves21m
- Velocity of Longitudinal Waves11m
- Wave Functions31m
- Phase Constant14m
- Average Power of Waves on Strings10m
- Wave Intensity19m
- Sound Intensity13m
- Wave Interference8m
- Superposition of Wave Functions3m
- Standing Waves30m
- Standing Wave Functions14m
- Standing Sound Waves12m
- Beats8m
- The Doppler Effect7m
- 19. Fluid Mechanics2h 27m
- 20. Heat and Temperature3h 7m
- Temperature16m
- Linear Thermal Expansion14m
- Volume Thermal Expansion14m
- Moles and Avogadro's Number14m
- Specific Heat & Temperature Changes12m
- Latent Heat & Phase Changes16m
- Intro to Calorimetry21m
- Calorimetry with Temperature and Phase Changes15m
- Advanced Calorimetry: Equilibrium Temperature with Phase Changes9m
- Phase Diagrams, Triple Points and Critical Points6m
- Heat Transfer44m
- 21. Kinetic Theory of Ideal Gases1h 50m
- 22. The First Law of Thermodynamics1h 26m
- 23. The Second Law of Thermodynamics3h 11m
- 24. Electric Force & Field; Gauss' Law3h 42m
- 25. Electric Potential1h 51m
- 26. Capacitors & Dielectrics2h 2m
- 27. Resistors & DC Circuits2h 7m
- 28. Magnetic Fields and Forces2h 23m
- 29. Sources of Magnetic Field2h 30m
- Magnetic Field Produced by Moving Charges10m
- Magnetic Field Produced by Straight Currents27m
- Magnetic Force Between Parallel Currents12m
- Magnetic Force Between Two Moving Charges9m
- Magnetic Field Produced by Loops and Solenoids42m
- Toroidal Solenoids aka Toroids12m
- Biot-Savart Law (Calculus)18m
- Ampere's Law (Calculus)17m
- 30. Induction and Inductance3h 37m
- 31. Alternating Current2h 37m
- Alternating Voltages and Currents18m
- RMS Current and Voltage9m
- Phasors20m
- Resistors in AC Circuits9m
- Phasors for Resistors7m
- Capacitors in AC Circuits16m
- Phasors for Capacitors8m
- Inductors in AC Circuits13m
- Phasors for Inductors7m
- Impedance in AC Circuits18m
- Series LRC Circuits11m
- Resonance in Series LRC Circuits10m
- Power in AC Circuits5m
- 32. Electromagnetic Waves2h 14m
- 33. Geometric Optics2h 57m
- 34. Wave Optics1h 15m
- 35. Special Relativity2h 10m
Moles and Avogadro's Number - Online Tutor, Practice Problems & Exam Prep
 Created using AI
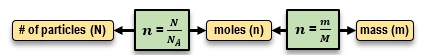
Created using AIIn thermodynamics, understanding the relationship between mass, moles, and the number of particles is crucial. A mole, represented by n, corresponds to Avogadro's number, approximately 6.022 × 1023 particles. The equations n = NNa and m = nM facilitate conversions between these quantities, enabling calculations of mass, moles, and particle counts effectively.
Moles & Avogadro's Number
Video transcript
If the molar mass of hydrogen is 1.008 g/mol, what is the mass (in grams) of 2 hydrogen atoms?
Calculating number of water molecules in a bottle
Video transcript
Hey, guys. Welcome back. Let's get started here. We have the molar mass of water, which is 18 grams per mole. But what we're asked to calculate in this problem is how many molecules of H2O we would find in a 1.5 liter bottle of water like one that you would find at the store. So let's get started here. Which variable are we asked to solve for? Well, in our flowcharts of particles, moles, and mass, we're actually looking for n, the number of particles or molecules. So really what I'm looking for is n of H2O. How many H2O molecules? So in order to find out big N, I'm gonna have to use this first equation here, the one that relates moles to particles by using Avogadro's number. So this little n is gonna be the number of H2O molecules divided by Avogadro's number. If you ever forget it, it's right here, \( 6.022 \times 10^{23} \). So, basically, I'm gonna have to move this over to the other side. I've got n times big N or that's that's Avogadro's number, sorry, and that's gonna be equal to the number of H2O molecules. Now I know what this is. This is just a constant. What I don't have though is the number of moles. All I'm told in this problem is that the molar mass is 18 grams per mole, but remember that is big M, that's capital M, that's the molar mass. And I'm only just told that this is a 1.5-liter bottle of water. I'm not told the number of moles. So I'm kind of stuck here, but it's okay because if I'm stuck on the number of moles, I can always get that by using the other equation, the one that relates mass to moles by using the molar mass. So, basically, that's what I have to do. I have to come over to this equation to solve for n. So that's n equals little m over big M. So really to find out the number of moles I just need the number of the mass divided by the molar mass. Now, I have the molar mass that's just the 18 grams per mole. What I don't have is the mass. All I'm told is the liters. Remember, that's just a unit of volume. Well, one thing you need to know, one conversion that's pretty useful to know is that 1 liter of water is equal to 1 kilogram of water. That's just a density thing. So I just kind of wanna put it here that this is only for water, but 1 liter is equal to 1 kilogram. So when it says a 1.5-liter bottle of water, really what this means is that m is equal to 1.5 kilograms. And now we have what the mass is equal to. So this little n is equal to 1.5 kilograms divided by, this is gonna be 18 grams per mole. Now just be very careful when you plug this into a calculator, you don't just go ahead and do this right now because we have kilograms and then grams per mole. So we need to make the units agree with each other. Either we can convert this unit to kilograms per mole or we can just expand this out to grams. Either one is perfectly acceptable. I think one is a little bit easier, just expanding the 1.5 kilograms to grams by moving the decimal place to the right. So 1.5 kilograms is 1500 divided by 18 grams per mole, which you'll see is that the grams cancel out and you're gonna get 83.3 moles. So that's how many moles that we're dealing with here, but that's not our final answer. Because remember we have to pop this back into this equation to solve for the number of particles. So basically, you're gonna take this and you just plug it back into for, for this little n here like this and what you're gonna get here is 83.3. This is gonna be moles, then you multiply by Avogadro's number. That's \( 6.022 \times 10^{23} \). And remember, this is particles per 1 mole. So what happens is the moles will cancel and you'll just end up with the number of particles, which is \( 5.02 \times 10^{25} \), and that's gonna be particles or let's say molecules of H2O. Alright. So that's it for this one. That's the final answer. Let me know if you have any questions.
Do you want more practice?
More setsHere’s what students ask on this topic:
What is Avogadro's number and why is it important in physics?
Avogadro's number, approximately 6.022 × 1023, represents the number of particles (atoms, molecules, etc.) in one mole of a substance. It is crucial in physics, especially in thermodynamics, because it allows for the conversion between the macroscopic scale (mass) and the microscopic scale (number of particles). This conversion is essential for understanding and calculating properties of gases, reactions, and other phenomena at the molecular level.
 Created using AI
Created using AIHow do you convert between moles and the number of particles?
To convert between moles (n) and the number of particles (N), you use Avogadro's number (NA). The equation is:
For example, if you have 1.2 × 1024 particles, the number of moles is:
So, you have approximately 1.99 moles.
 Created using AI
Created using AIHow do you calculate the mass of a substance from the number of moles?
To calculate the mass (m) of a substance from the number of moles (n), you use the molar mass (M) of the substance. The equation is:
For example, if you have 2.5 moles of aluminum (Al) with a molar mass of 26.98 g/mol, the mass is:
So, you have 67.45 grams of aluminum.
 Created using AI
Created using AIWhat is the relationship between mass, moles, and the number of particles?
The relationship between mass (m), moles (n), and the number of particles (N) is interconnected through Avogadro's number (NA) and the molar mass (M). The key equations are:
These equations allow you to convert between the number of particles, the amount of substance in moles, and the mass of the substance. For example, knowing the number of particles and Avogadro's number, you can find the moles, and using the molar mass, you can then find the mass.
 Created using AI
Created using AIHow do you find the number of particles in a given mass of a substance?
To find the number of particles (N) in a given mass (m) of a substance, you first convert the mass to moles (n) using the molar mass (M), and then convert moles to particles using Avogadro's number (NA). The steps are:
1. Calculate moles:
2. Calculate particles:
For example, for 24 grams of H2O (molar mass = 18.02 g/mol):
So, there are approximately 8.01 × 1023 particles of H2O.
 Created using AI
Created using AIYour Physics tutor
- How many atoms are in a 2.0 cm×2.0 cm×2.0 cm cube of aluminum?
- An element in its solid phase has mass density 1750 kg/m^3 and number density 4.39×10^28 atoms/m^3. What is th...
- (II) How many atoms are there in a 3.4-g copper coin?
- Calculate the number of molecules/m³ in an ideal gas at STP.
- (I) If 3.50 m³ of a gas initially at STP is placed under a pressure of 2.80 atm, the temperature of the gas ri...
- How many moles of water are there in 1.00 L at STP? How many molecules?
- (II) A storage tank contains 21.6 kg of nitrogen at an absolute pressure of 3.45 atm. What will the pressure b...
- (II) A tank contains 30.0 kg of O₂ gas at a gauge pressure of 8.20 atm. If the oxygen is replaced by helium at...
- "(II) Compare the value for the density of water vapor at exactly 100°C and 1 atm (Table 13–1) with the value ...
- (II) What is the pressure in a region of outer space where there is 1 molecules/m³ and the temperature is 3 K?
- Estimate the number of air molecules in a room of length 6.0 m, width 3.0 m, and height 2.5 m. Assume the temp...
- "(II) Estimate the number of(a) moles. Assume water covers 75% of the Earth to an average depth of 3 km."
- (II) Estimate the number of(b) molecules of water in all the Earth’s oceans.Assume water covers 75% of the Ear...
- How does the number of atoms in a 31.5-g gold ring compare to the number in a silver ring of the same mass?
- (II) Calculate the density of nitrogen at STP using the ideal gas law.
- From the known value of atmospheric pressure at the surface of the Earth, estimate the total number of air mol...