Pressure gauges are essential devices used to measure pressure, with the barometer being a specific type that measures atmospheric pressure. The fundamental principle behind pressure measurement involves height differences and the densities of liquids, which can be expressed using the equation:
\[ P_{\text{bottom}} = P_{\text{top}} + \rho g h \]
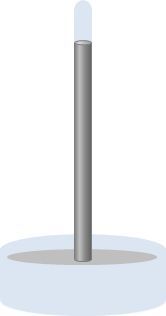
In the context of a barometer, the setup typically includes a container filled with a heavy liquid, such as mercury, and a glass tube. The bottom of the container is open to the atmosphere, allowing air pressure to exert force on the liquid, causing it to rise in the tube. The top of the tube is sealed, creating a vacuum. At equilibrium, the height of the liquid column reflects the atmospheric pressure, leading to a simplified equation for barometers:
\[ P_{\text{air}} = \rho g h \]
Here, \( P_{\text{air}} \) represents the atmospheric pressure, \( \rho \) is the density of the liquid, \( g \) is the acceleration due to gravity, and \( h \) is the height of the liquid column. This equation indicates that to calculate atmospheric pressure, one needs to know the density of the liquid and measure the height of the liquid column.
Standard atmospheric pressure at sea level is defined as 1 atmosphere (atm), which corresponds to a height of approximately 76 cm of mercury. However, this value can change with altitude; for instance, at higher elevations, the atmospheric pressure decreases, resulting in a lower height of the liquid column in the barometer.
Mercury is commonly used in barometers due to its high density (approximately 13.6 times that of water), which allows for a more compact design. For example, if water were used instead, the height of the column would need to be about 10.3 meters to equal 1 atm, making the instrument impractical.
To illustrate the application of these principles, consider a barometer filled with an unknown liquid that rises to 76 cm under standard atmospheric pressure. By rearranging the equation to solve for density, we find:
\[ \rho = \frac{P_{\text{air}}}{g h} \]
Substituting \( P_{\text{air}} = 101,000 \, \text{Pa} \), \( g = 9.8 \, \text{m/s}^2 \), and \( h = 0.76 \, \text{m} \), we calculate the density to be approximately 13,560 kg/m³, which corresponds to the density of mercury.
In a different scenario, if the same barometer is taken to a location where the liquid rises to 84 cm, we can again use the equation to find the new atmospheric pressure:
\[ P_{\text{air}} = \rho g h \]
Using the previously determined density of mercury, we substitute the values to find that the atmospheric pressure is approximately 11,625 Pa, or about 1.1 atm. This increase in height indicates that the external air pressure is greater, pushing the liquid higher in the tube.
Understanding how barometers function and the relationship between liquid height, density, and atmospheric pressure is crucial for various applications in meteorology and environmental science.