21. Kinetic Theory of Ideal Gases
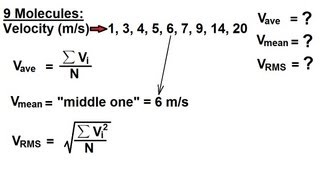
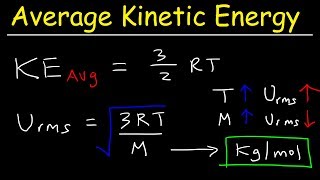
Root-Mean-Square Velocity of Gases
21. Kinetic Theory of Ideal Gases
Root-Mean-Square Velocity of Gases
Additional 4 creators.
Learn with other creators
Showing 7 of 7 videos
Practice this topic
- Multiple Choice
What is the temperature of a sample of CO2 molecules whose rms speed is 300 m/s? The molecular mass for Carbon and Oxygen is 12.01 g/mol and 16 g/mol, respectively.
576views3rank - Textbook QuestionOxygen (O2) has a molar mass of 32.0 g/mol. What is (g) How many oxygen molecules traveling at this speed are necessary to produce an average pressure of 1 atm?1169views
- Textbook QuestionOxygen (O2) has a molar mass of 32.0 g>mol. What is (e) Suppose an oxygen molecule traveling at this speed bounces back and forth between opposite sides of a cubical vessel 0.10 m on a side. What is the average force the molecule exerts on one of the walls of the container? (Assume that the molecule's velocity is perpendicular to the two sides that it strikes.)737views
- Textbook QuestionFor diatomic carbon dioxide gas (CO2, molar mass 44.0 g/mol) at T = 300 K, calculate (c) the root-mean-square speed v_rms.966views
- Textbook QuestionSmoke particles in the air typically have masses of the order of 10-16 kg. The Brownian motion (rapid, irregular movement) of these particles, resulting from collisions with air molecules, can be observed with a microscope. (a) Find the rootmean-square speed of Brownian motion for a particle with a mass of 3.00 * 10-16 kg in air at 300 K.1602views