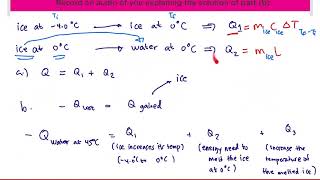
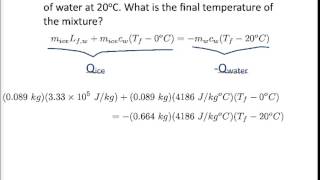
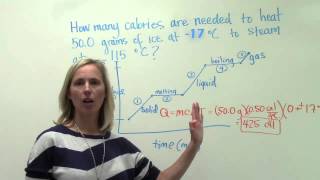20. Heat and Temperature
Advanced Calorimetry: Equilibrium Temperature with Phase Changes
Learn with other creators
Practice this topic
- Textbook Question
What will be the final result when equal masses of ice at 0°C and steam at 100°C are mixed together?
220views - Textbook Question
(II) What mass of steam at 100°C must be added to 1.00 kg of ice at 0°C to yield liquid water at 30°C?
244views - Textbook Question
(II) Determine the latent heat of fusion of mercury using the following calorimeter data: 1.00 kg of solid Hg at its melting point of −39.0°C is placed in a 0.620-kg aluminum calorimeter with 0.400 kg of water at 12.80°C; the resulting equilibrium temperature is 5.06°C.
169views - Textbook Question
Before going in for his annual physical, a 70.0-kg man whose body temperature is 37.0°C consumes an entire 0.355-L can of a soft drink (mostly water) at 12.0°C. (a) What will his body temperature be after equilibrium is attained? Ignore any heating by the man’s metabolism. The specific heat of the man’s body is 3480 J/kg K.
154views