Back
24. Electric Force & Field; Gauss' Law - Part 1 of 2
24. Electric Force & Field; Gauss' Law - Part 1 of 2
24. Electric Force & Field; Gauss' Law / Electric Charge / Problem 2
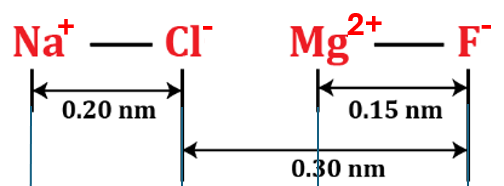
Consider a scenario involving two ion pairs: NaCl and MgF. The charges of the ions are as follows: Na+ and Cl− have charges +1.00𝑒 and -1.00𝑒, respectively. On the other hand, Mg2+ and F− have charges +2.00𝑒 and -1.00𝑒, respectively, where 𝑒=1.602×10−19 C. Calculate the net electrostatic force between the NaCl ion pair and the MgF ion pair, considering the positive direction to be to the right. Hint: Ignore the forces between ions of the same pair.
Was this helpful?

Learn this concept