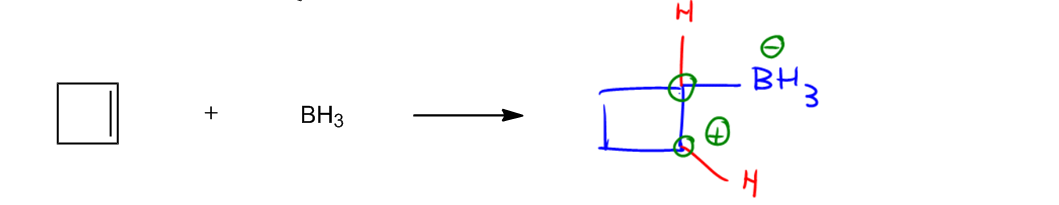
Substitution reactions in organic chemistry involve the interaction between nucleophiles and electrophiles, where electrons move predictably from areas of high electron density to areas of low electron density. Nucleophiles, which are typically negatively charged species, attack electrophiles, which are electron-deficient. This fundamental principle of electron movement is crucial for understanding various organic reactions.
In substitution reactions, the nucleophile replaces a leaving group attached to the electrophile. The nature of the nucleophile and the electrophile, as well as the conditions of the reaction, can lead to different types of substitution mechanisms, such as SN1 and SN2. In an SN2 reaction, for instance, the nucleophile attacks the electrophile in a single concerted step, resulting in the simultaneous displacement of the leaving group. Conversely, in an SN1 reaction, the process occurs in two steps: first, the leaving group departs, forming a carbocation, followed by the nucleophile's attack on this carbocation.
Understanding these mechanisms is essential for predicting the outcomes of substitution reactions and for applying this knowledge to synthesize various organic compounds. The ability to identify nucleophiles and electrophiles, along with their reactivity patterns, is a key skill in organic chemistry.
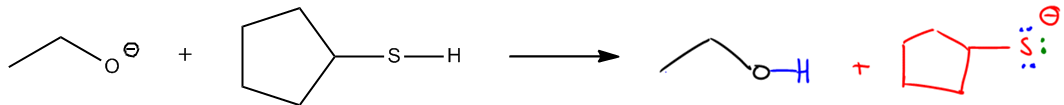

 1 student found this helpful
1 student found this helpful