For this section, I'm going to be introducing a completely new type of organic reaction, and that's the condensation reaction. Whenever you get 2 molecules that spontaneously combine to form a bigger molecule with the loss of a smaller molecule on the side, that's what's called a condensation reaction. There are actually tons of different types of condensation reactions in organic chemistry. But the specific ones that we'll be dealing with in this section have to do with enolates. Remember that enolates are the negative anions of a deprotonated alpha carbon. What we find is that enolates are going to be part of the condensation because enolates are so reactive that they're not only just going to react with electrophiles as we've talked about in the past. They're also even going to be able to react with each other or with themselves to do something that's called self-condensation. Obviously, due to the fact that there's an enolate mediated reaction, alpha protons are required for this type of reaction to take place because if you don't have an alpha proton, then you're not getting an enolate because an enolate is the deprotonated version of an alpha carbon.
I want to just back it up for a second and go back to what we know and use that information to inform our understanding of condensation reactions. Back in the day, it seems like the glory days now. We learned about nucleophilic addition. We learned that carbonyls were especially reactive at the carbonyl carbon. When nucleophiles would attack, we would get a mechanism called nucleophilic addition where a tetrahedral intermediate would protonate, and we would get an alcohol as a result. Fair. We did that a lot. More recently, what we've learned is that certain types of nucleophiles, specifically basic ones, are going to react at the alpha carbon and take away a proton. We've noticed that, for example, O- can remove an alpha proton and make what we call an enolate anion, a negative charge on that alpha carbon. What's special about that negatively charged enolate is that now we know that it can attack electrophiles. It could attack some alkyl halide or some halogen, and we can get an alpha substituted carbonyl. Good. Awesome.
What we're going to learn in this section is how enolates relate to condensation reactions. It turns out, the same thing when a base deprotonates an alpha carbon, you're going to get an enolate. But what happens when you don't have an electrophile to react with? What happens in the absence of an electrophile? What if I just make my enolate but then there's nothing to react it with? I never add that other alkyl halide or that halogen. What do we do? In that special case where there is no electrophile, then the enolate is going to react with itself. What we're going to find is that the enolate is going to react with the non-enolated version of itself. It's going to do a nucleophilic addition, making a tetrahedral intermediate on the other ketone. Isn't that interesting? Basically, this acts as the nucleophile for another nucleophilic addition. What we wind up getting is a condensation reaction because now I'm taking 2 ketones or aldehydes. I'm combining them together into 1 larger molecule. Notice what I would get here just for this tetrahedral intermediate. What I would get is now RA here, received a negative charge that would eventually be protonated. This is the product of enolate condensation. This is what we're going to be focusing on for quite a few videos. We're going to be spending a lot of time on enolate condensation.
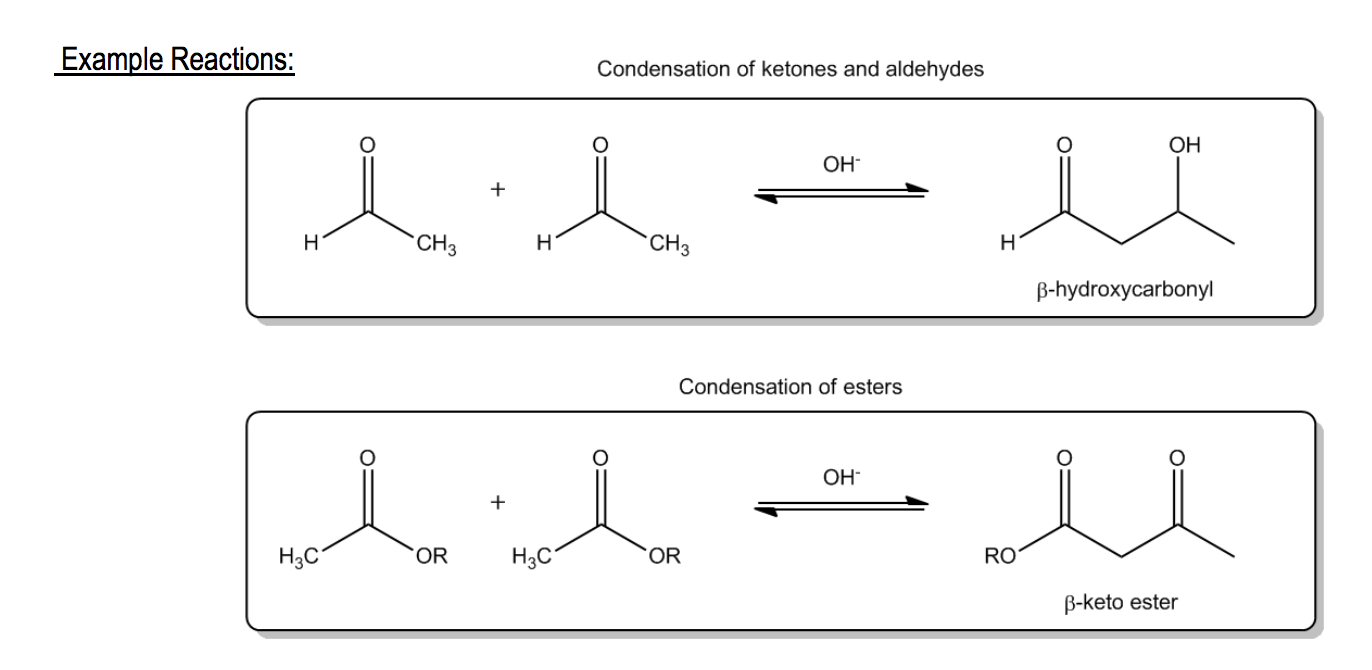
I want to give you guys some examples. This is going to kind of be a preview of what we're going to be learning in a little bit. It turns out that ketones and aldehydes can combine together to make molecules that have alcohol components and carbonyl. This reaction in particular is called an aldol reaction. The reason being that, as we'll see more later, there's part alcohol and there's part aldehyde. It's an aldol reaction. Notice this is condensation because I've got 2 smaller molecules combined to form a bigger one. Another example, what if we do this with 2 esters? We take 2 esters. We make an enolate. They react with each other. They're going to make a beta dicarbonyl, specifically a beta ketoester. This reaction is called a Claisen condensation. This is a preview. You're not supposed to learn these here. I'm just trying to show you guys how we're going to be dealing with a lot of reactions that condensate with enolates. These are 2 of the most important, but we're going to deal with a lot more in this section. That being said, I hope the general mechanism makes sense so far. Let's move on to our first condensation reaction.