Hey everyone. On this next page, we're going to define 2 extremely important concepts that are going to be essential for this next topic. The 2 things we're going to define are what a carboxylic acid derivative is, a category of molecule, and the mechanism that it undergoes, NAS or nucleophilic acyl substitution. Let's start off with the first question. What is a carboxylic acid derivative? It's simply defined as any carbonyl that has an electronegative Z group in the alpha position. It's kind of a lot to chew on. Let's break that down.
Remember that in ketones and aldehydes, by definition, you're stuck with an R or an H. Now, R and H are not electronegative at all. In fact, they make terrible leaving groups. If you think about H negative, that's a strong base; R negative, that's an even stronger base. These are not good leaving groups. What we find is that there's a certain mechanism that they tend to undergo, which we'll see in a second. Whereas, Z groups are defined as something that's slightly electronegative even to very electronegative.
Here are all the Z groups that we're going to be working with in this section. We've got chlorine. We've got basically an ester, OR-OH, NH2. That's a typo. That should actually be NH2-. Sorry about that. Even tutors make mistakes sometimes. Those are our Z groups. Now notice that they're not all quite as good as electronegative groups. For example, chlorine is very electronegative, while nitrogen, not so much. But the reason that we cluster them all together is because they are much better than R groups and hydrogen no matter what.
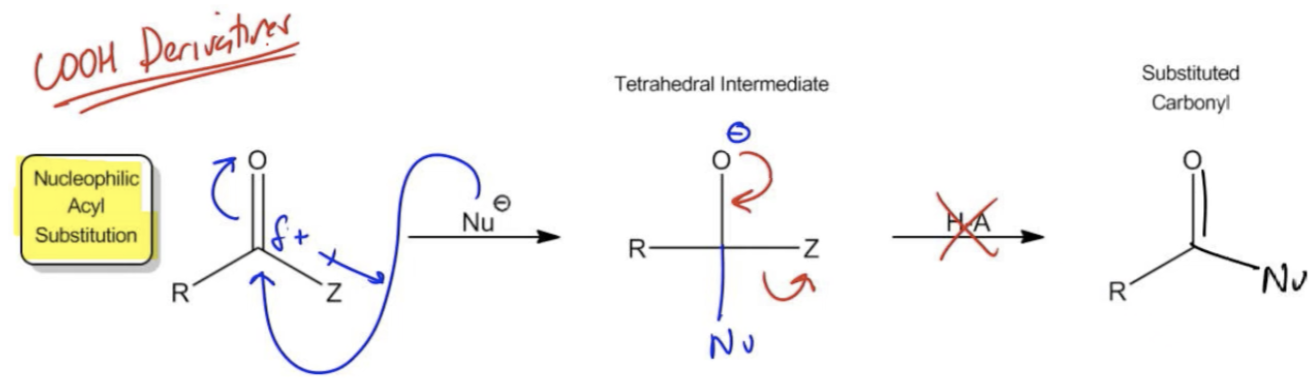
It turns out that by definition, these Z groups are going to allow these carbonyls to follow a new mechanism that's different from the mechanism that we would see in a ketone and aldehyde called NAS or nucleophilic acyl substitution. A few more definitions about carboxylic acid derivatives: By definition, anything that we call a carboxylic acid derivative can be hydrolyzed back to carboxylic acid using a combination of water with acid or base.
If I ever tell you that this is a carboxylic acid derivative, that is me saying that you could use water to hydrolyze it back to carboxylic acid which, as you see, carboxylic acid would be if I used an OH-. Carboxylic acid is also a Z group; it's just a specific one. Carboxylic acid, you could think of as the mother of all of the other carboxylic acid derivatives because you can always turn those derivatives back into carboxylic acid with hydrolysis.
Another really interesting fact here is that nitriles also fall into this category due to their ability to be hydrolyzed. We're going to see later that notice that I don't have nitrile on my list but nitriles look like this, carbon-nitrogen like that. It turns out that they can be hydrolyzed using basically water and acid or base to carboxylic acids. We consider nitriles to also be carboxylic acid derivatives.
In this next video, what I'm going to do is show you guys the differences between nucleophilic addition, which is the mechanism that ketones and aldehydes undergo, versus nucleophilic acyl substitution, which is what carboxylic acid derivatives undergo.