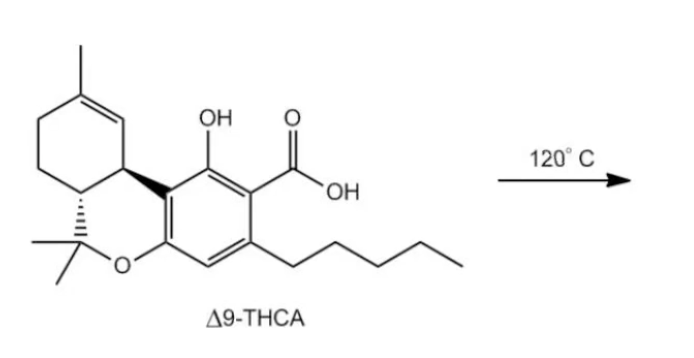
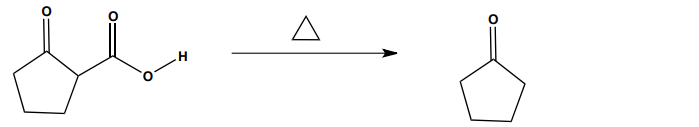
Decarboxylation is a crucial reaction in organic chemistry, particularly for the transformation of carboxylic acids. This process involves the removal of a carboxyl group (-COOH) from a beta carbonyl carboxylic acid when heated, resulting in the release of carbon dioxide (CO2) gas as a byproduct. A beta carbonyl carboxylic acid is characterized by having a carbonyl group (C=O) adjacent to the carboxylic acid group.
The mechanism of decarboxylation can be understood through a series of steps involving the movement of electrons, often illustrated with arrow notation. Initially, the oxygen atom of the hydroxyl group in the carboxylic acid abstracts a hydrogen atom (H), forming a bond and creating a pi bond between the carbon and the oxygen. This action leads to the cleavage of the bond connecting the carboxylic acid to the alkyl group, resulting in the formation of CO2 gas and leaving behind a carbonyl compound.
After the decarboxylation process, the product may initially appear as an alcohol (vinyl alcohol), which is a common point of confusion. However, vinyl alcohols are known to undergo tautomerization, a process where the position of a double bond and a hydrogen atom are rearranged. This transformation leads to the formation of a more stable keto form, specifically a ketone. Understanding this transition is essential, as enol and enolate chemistry plays a significant role in various organic reactions.
In summary, decarboxylation is a heat-mediated reaction that effectively removes carboxylic acid groups, producing carbon dioxide and resulting in the formation of carbonyl compounds through tautomerization. Mastery of this reaction and its mechanisms is vital for further studies in organic chemistry.