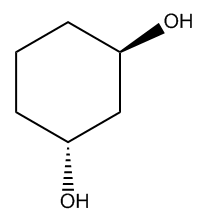
Understanding the naming of alcohols is essential in organic chemistry, particularly when dealing with functional groups. Alcohols are characterized by the presence of one or more hydroxyl groups (-OH) attached to a carbon chain. When a molecule contains more than one hydroxyl group, it is referred to as a glycol, although this term is quite general. To provide more specificity, we use prefixes that indicate the number of hydroxyl groups present.
For instance, an alcohol with two hydroxyl groups is termed a diol, while one with three hydroxyl groups is called a triol. This naming convention continues with prefixes such as tetra- for four hydroxyl groups, and so on. It is crucial to remember that when numbering the carbon chain or ring, the hydroxyl group receives the highest priority. This principle is often summarized by the phrase "alcohol beats all," indicating that the presence of an alcohol group takes precedence over other functional groups, such as double bonds, triple bonds, or alkyl halides, when determining the numbering of the compound.
In practice, this means that when you encounter a molecule with multiple functional groups, you should always prioritize the hydroxyl group in your numbering scheme. This foundational knowledge will aid in accurately naming and understanding the structure of various alcohols and their derivatives.