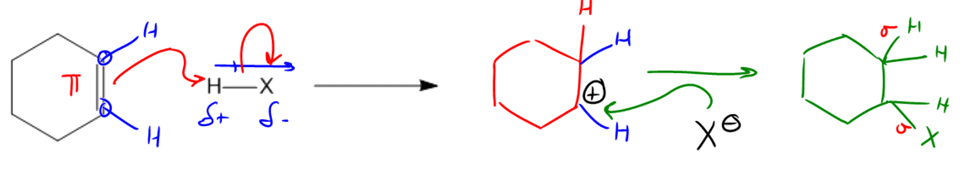
In organic chemistry, a fundamental concept is the interaction between nucleophiles and electrophiles, which can be effectively summarized through addition reactions. These reactions involve the breaking of a pi bond and the formation of two new sigma bonds, thereby increasing the total number of bonds in the molecule. This process is termed "addition" because it adds to the bonding framework of the compound.
The mechanism of an addition reaction typically begins with a nucleophile, which is often a double bond due to its electron-rich nature. The double bond acts as the source of electrons, initiating the reaction by forming an arrow that points towards the electrophile, which is the positively charged or electron-deficient species. In many cases, this electrophile can be a hydrogen atom (H) when reacting with a halogen (X), where X represents any halogen atom.
To identify the electrophilic portion, one can analyze the dipoles within the molecule. For instance, in a molecule containing H and X, the halogen is more electronegative than hydrogen, resulting in a partial positive charge on the hydrogen and a partial negative charge on the halogen. Thus, the nucleophile (the double bond) will direct its electrons towards the hydrogen atom.
Upon forming a bond with the hydrogen, the double bond is broken, leading to the creation of a carbocation, which is a carbon atom that has only three bonds instead of the usual four, indicating it is electron-deficient. This carbocation can then react with a negatively charged species, such as X-, which will donate its electrons to the carbocation, completing the octet of the carbon atom.
The final product of this addition reaction will consist of two hydrogen atoms and one halogen atom attached to the carbon framework, demonstrating the transformation from one pi bond to two sigma bonds. This mechanism is crucial as it lays the groundwork for understanding various organic reactions that will be explored throughout the course.