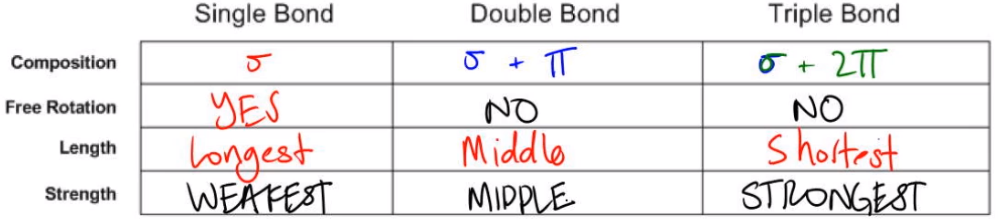
In the study of chemical bonding, understanding the types of bonds—single, double, and triple—is essential. Each bond type has distinct characteristics based on its composition, free rotation, length, and strength.
A single bond consists of one sigma bond, which is formed by the head-on overlap of atomic orbitals. This bond allows for free rotation because there is only one region of overlap, enabling the atoms to rotate around the bond axis without breaking the bond. In terms of length, single bonds are the longest among the three types, and they are also the weakest, as they have only one overlapping region that contributes to bond stability.
A double bond is composed of one sigma bond and one pi bond. The sigma bond provides a central region of overlap, while the pi bond involves the side-to-side overlap of p orbitals. This structure restricts rotation; attempting to rotate one of the atoms would disrupt the pi bond, making it energetically unfavorable. Double bonds are shorter than single bonds but longer than triple bonds, and they possess intermediate strength, being stronger than single bonds but weaker than triple bonds.
A triple bond consists of one sigma bond and two pi bonds. The presence of two pi bonds results in three regions of overlap, making triple bonds the shortest and strongest type of bond. Similar to double bonds, triple bonds do not allow for free rotation due to the need to maintain the integrity of the pi bonds. The energy saved by a triple bond is greater than that of a double bond, which in turn is greater than that of a single bond.
In summary, the hierarchy of bond types can be understood as follows: single bonds are the longest and weakest, double bonds are intermediate in both length and strength, and triple bonds are the shortest and strongest. The sigma bond is the strongest type of bond due to its effective overlap, while pi bonds contribute additional strength but are less effective individually. This understanding of bond types is crucial for predicting molecular behavior and reactivity.