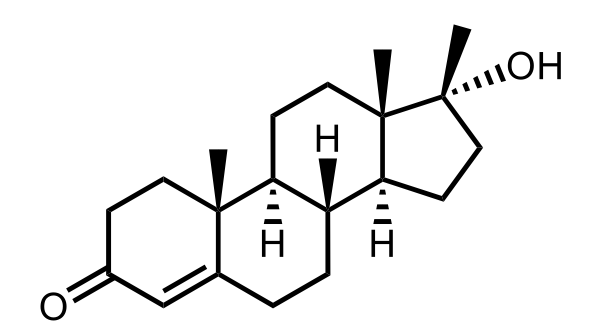
If we're drawing steroids, we need to realize that drawing a steroid requires knowledge of some common stereochemical features along with given information. In this example, it says, draw on the structure of testosterone using the information given below. Here, we need a carbonyl group at carbon number 3. We need a double bond between carbons C4 and C5. And, finally, a beta hydroxyl group at carbon 17. Alright. So, step 1 is we're going to start by drawing the steroid skeleton which is comprised of 3 cyclohexane rings and 1 cyclopentane ring, and we have to number all the carbons. Alright. So here, we have just the skeletal outline of this. Alright. So here is our structure to begin. And if we wanted to number this structure, right, we'd say that this is 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10. And this would be, it is going to be here. If we wanted to continue counting, we have to go to the other side here. So now, this would be 11, 12, 13, 14, 15, 16, 17. If there were alkyl groups attached to 17, then that would be carbon 18. If there's an alkyl group attached to carbon 10, that would be 19. Alright. So this is our numbered structure. Now we're gonna go to step 2 here. It says draw the 2 methyl groups at Carbon 13 and carbon 10 using wedges. Remember, wedges here would mean up which mean they take on the beta configuration. So here, let's add that in now. We're using the top one as a reference. Alright. So carbons 13 and 10, well, actually, it would be carbons 13 and 10 where we would go, 18 and 19. So here, we're gonna say here let's see. We have to add 2 methyl groups. So one here, wedged, and one here, wedged. Alright. So for C13, this would be carbon 18 and 19 respectively. Now, next, we have to draw the C5 hydrogens using dashes or we have trans alpha beta junction unless otherwise stated. Alright. So we're gonna continue drawing. So at C5 we have our hydrogen which is going to be dashed. Then next, moving right from C10, we have our methyl group. We're gonna draw hydrogen atoms on the junction as alpha which is down, beta which is up, and then alpha which is down again. So coming back up here, we're going to do that. Alright. So C10, moving right from C10, right from C10. We're gonna have here carbon 9. There's gonna be an H that's dashed for alpha and then one that's up for beta, and then one that's dashed for alpha. So that's what we have so far. And again, these are our methyl groups. Now, fill in all the given information to complete the structure. Alright. So we're told that carbon number 3 has a carbonyl group. So here goes our carbonyl group. Carbons 4 and 5, well, they're gonna have a pi bond, which would mean I have to get rid of this hydrogen here because carbon can't make more than 4 bonds. And then it told us what else? Oh, a beta hydroxyl OH group on carbon number 17. So beta would mean that it's pointing up. So there goes the OH, and it's pointing up. So this would be the structure. We drew out the base steroid skeletal structure, and from there, we added the, essentially, the modifications from the example question itself. We added a carbonyl group on carbon 3, a pi bond between carbons 4 and 5, and then finally, a beta hydroxyl on carbon number 17 to give us this final depiction of our compound.