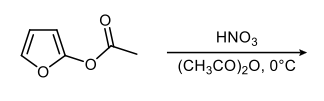
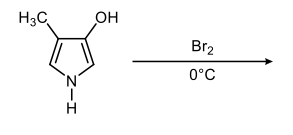
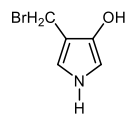
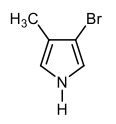
In the study of heterocyclic compounds, particularly five-membered aromatic heterocycles, understanding the directing effects of substituents is crucial. The terms ortho, meta, and para, commonly used in the context of substituted benzene rings, also apply to these heterocycles, allowing for a comparative analysis of their substituent positions.
When discussing substituents, it is essential to assign their positions based on the carbon skeleton rather than the heteroatom. For instance, in a heterocycle with two methyl groups positioned ortho to each other, they can be labeled as positions 1 and 2. This parallels the nomenclature used for substituted benzene rings, where the substituents maintain their ortho relationship.
Similarly, when examining a heterocycle with a carboxylic acid and a nitrile group, these substituents are positioned meta to each other, corresponding to positions 1 and 3. This relationship mirrors that of substituted benzene, reinforcing the concept of directing effects across different types of aromatic systems.
Lastly, in a scenario where two bromine atoms are positioned para to each other, they are designated as positions 1 and 4. This consistent numerical relationship among substituents on heterocycles aids in applying directing effects to both the heteroatom and the substituents in future reactions.
Overall, while the naming conventions for heterocycles may differ, the principles governing the directing effects of substituents remain fundamentally similar to those observed in benzene derivatives, providing a valuable framework for understanding their reactivity and interactions.