Back
15. Analytical Techniques:IR, NMR, Mass Spect - Part 2 of 4
15. Analytical Techniques:IR, NMR, Mass Spect - Part 2 of 4
15. Analytical Techniques:IR, NMR, Mass Spect / 1H NMR:Q-Test / Problem 6
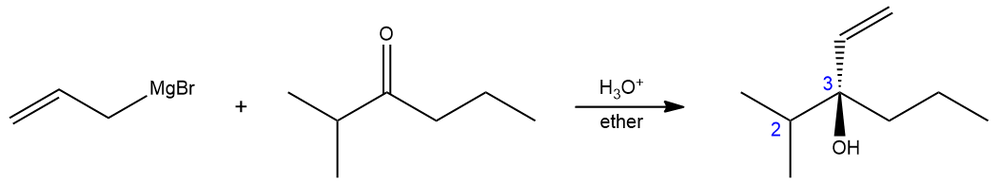
In an organic chemistry lab experiment, 2-methyl-3-hexanone is reacted with allyl magnesium bromide yielding a tertiary alcohol. The 1H NMR of 2-methyl-3-hexanone shows that the two methyl groups are equivalent indicated by a one doublet peak. On the other hand, the product (a racemic mixture), gives two different 3H doublets.
(i) Along the C2–C3 axis, show a Newman projection of the product.
(ii) Why do the methyl groups of the product show different signals? What is the term used to describe such groups?
Was this helpful?

Learn this concept