Here are the essential concepts you must grasp in order to answer the question correctly.
Nucleophilic Substitution Reactions
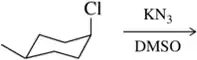
Nucleophilic substitution reactions involve the replacement of a leaving group in a molecule by a nucleophile. These reactions can occur via two main mechanisms: SN1, which is a two-step process involving carbocation formation, and SN2, which is a one-step process where the nucleophile attacks the substrate simultaneously as the leaving group departs. Understanding these mechanisms is crucial for predicting the products and stereochemical outcomes of the reactions.
Recommended video:
Nucleophiles and Electrophiles can react in Substitution Reactions.
Stereochemistry
Stereochemistry refers to the study of the spatial arrangement of atoms in molecules and how this affects their chemical behavior. In substitution reactions, the stereochemical outcome can vary significantly depending on whether the reaction follows an SN1 or SN2 mechanism. For example, SN2 reactions result in inversion of configuration at the chiral center, while SN1 reactions can lead to racemization due to the formation of a planar carbocation intermediate.
Recommended video:
Polymer Stereochemistry Concept 1
Leaving Groups
Leaving groups are atoms or groups that can depart from the parent molecule during a chemical reaction, facilitating nucleophilic substitution. The ability of a leaving group to stabilize the negative charge after departure is critical; good leaving groups, such as halides or sulfonate esters, enhance the reaction rate. Identifying the nature of the leaving group is essential for predicting the feasibility and outcome of substitution reactions.
Recommended video:
The 3 important leaving groups to know.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 3:32m
3:32m