Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Shift in NMR Spectroscopy
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift refers to the resonance frequency of a nucleus relative to a standard in a magnetic field. It is influenced by the electronic environment surrounding the nucleus, with electronegative atoms or double bonds causing deshielding, leading to a higher frequency signal. Understanding chemical shifts is crucial for predicting and interpreting NMR spectra.
Recommended video:
Electronegativity and Its Effect on NMR
Electronegativity is the tendency of an atom to attract electrons towards itself. In NMR, electronegative atoms (like F or O) can pull electron density away from nearby hydrogen atoms, causing those hydrogens to experience a greater magnetic field and resonate at a higher frequency. This concept is essential for determining which hydrogens will appear at higher frequencies in an NMR spectrum.
Recommended video:
Alkenes vs. Alkanes in NMR
Alkenes, which contain carbon-carbon double bonds, typically show different NMR signals compared to alkanes, which are saturated hydrocarbons. The presence of a double bond can lead to deshielding effects on adjacent hydrogens, resulting in higher frequency signals. Recognizing the structural differences between alkenes and alkanes is vital for predicting their NMR behavior.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: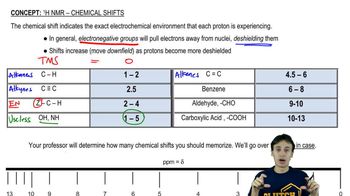


 11:44m
11:44m