Here are the essential concepts you must grasp in order to answer the question correctly.
Lewis Structures
Lewis structures are diagrams that represent the bonding between atoms in a molecule and the lone pairs of electrons that may exist. They help visualize the arrangement of electrons and the connectivity of atoms, which is crucial for understanding molecular geometry and reactivity. Drawing a Lewis structure involves determining the total number of valence electrons and distributing them to satisfy the octet rule for each atom.
Recommended video:
Drawing the Lewis Structure for N2H4.
Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. They determine the properties and reactivity of organic compounds. In the provided structure, the presence of a carbonyl group (C=O) and an amine group (N-H) indicates that the compound may exhibit properties typical of ketones or amides, respectively.
Recommended video:
Identifying Functional Groups
Molecular Classification
Molecular classification involves categorizing compounds based on their structural features and functional groups. This classification helps predict the behavior and reactivity of the compounds. For example, the compound in the image can be classified as an amide due to the presence of the carbonyl group adjacent to a nitrogen atom, which influences its chemical properties and potential reactions.
Recommended video:
Review of Molecular Orbitals


 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: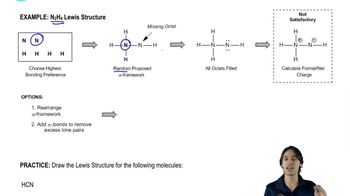



 1:49m
1:49m