Here are the essential concepts you must grasp in order to answer the question correctly.
Chirality and Stereoisomers
Chirality refers to the property of a molecule that makes it non-superimposable on its mirror image, leading to the existence of stereoisomers. (S)-2-butanol is a chiral molecule, meaning it has a specific three-dimensional arrangement that can exist in two enantiomeric forms. Understanding chirality is essential to grasp how reactions involving chiral centers can lead to the formation of racemic mixtures.
Recommended video:
Racemic Mixture
A racemic mixture is a 1:1 mixture of two enantiomers of a chiral compound, resulting in no optical activity. When (S)-2-butanol is heated in sulfuric acid, it can undergo dehydration to form a carbocation intermediate, which can then be attacked by nucleophiles from either side, leading to the formation of both (S)- and (R)-2-butanol. This process illustrates how racemic mixtures can arise from chiral starting materials.
Recommended video:
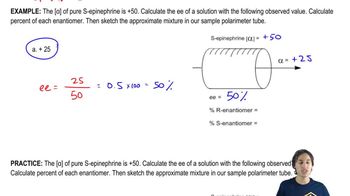
Calculating EE, percent of each enantiomer, and sketching mixture
Carbocation Stability and Rearrangement
Carbocations are positively charged species that can undergo rearrangements to form more stable structures. In the case of (S)-2-butanol, heating in sulfuric acid generates a carbocation that can rearrange or react in a way that allows for the formation of both enantiomers. The stability of the carbocation and the possibility of different pathways contribute to the racemic outcome of the reaction.
Recommended video:
Determining Carbocation Stability
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 6:01m
6:01m