Here are the essential concepts you must grasp in order to answer the question correctly.
Acid-Catalyzed Isomerization
Acid-catalyzed isomerization involves the rearrangement of molecular structures under acidic conditions, typically facilitated by protonation of a double bond. This process allows less stable alkenes to convert into more stable isomers by overcoming energy barriers through the formation of carbocation intermediates. The presence of sulfuric acid provides protons that enhance the reactivity of the alkene, enabling the isomerization to occur.
Recommended video:
Microscopic Reversibility
Microscopic reversibility is a principle stating that the mechanism of a reaction can be reversed at the molecular level. In the context of acid-catalyzed isomerization, this means that the steps leading to the formation of a more stable isomer can also be followed in reverse to regenerate the original alkene. This concept emphasizes the symmetry in chemical reactions, where the forward and reverse pathways share similar transition states.
Recommended video:
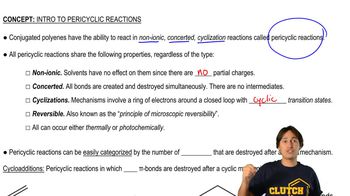
Properties and Types of Pericyclic Reactions
Carbocation Stability
Carbocation stability is a key factor in determining the outcome of reactions involving alkenes. Carbocations are positively charged intermediates that can vary in stability based on their structure; tertiary carbocations are more stable than secondary or primary ones due to hyperconjugation and inductive effects. Understanding the stability of carbocations is crucial for predicting the favored isomerization pathway and the final product in acid-catalyzed reactions.
Recommended video:
Determining Carbocation Stability

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: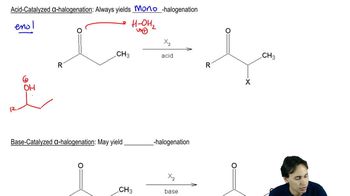


 6:01m
6:01m