Here are the essential concepts you must grasp in order to answer the question correctly.
Sigmatropic Rearrangement
Sigmatropic rearrangements are a class of pericyclic reactions where a sigma bond and a pi bond are rearranged in a concerted process. These reactions involve the migration of a substituent across a double bond, resulting in a structural change of the molecule. Understanding the mechanism and the types of sigmatropic rearrangements is crucial for predicting the products of such reactions.
Recommended video:
Nomenclature of Sigmatropic Shifts
Types of Sigmatropic Rearrangements
Sigmatropic rearrangements are categorized based on the number of atoms involved in the migration. The most common types are [1,3] and [1,5] rearrangements, where the numbers indicate the positions of the migrating atom relative to the original bond. Recognizing these types helps in identifying the specific rearrangement occurring in a given reaction.
Recommended video:
Nomenclature of Sigmatropic Shifts
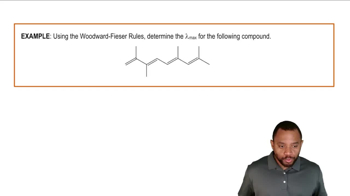
Woodward-Hoffmann Rules
The Woodward-Hoffmann rules provide a framework for predicting the stereochemical outcomes of pericyclic reactions, including sigmatropic rearrangements. These rules are based on the conservation of orbital symmetry and help determine whether a reaction is allowed or forbidden under thermal or photochemical conditions. Applying these rules is essential for understanding the feasibility of the rearrangements in the given reactions.
Recommended video:
Woodward-Fieser Rules Example 1


 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: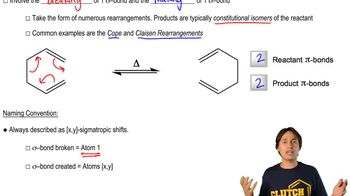


 3:51m
3:51m