Here are the essential concepts you must grasp in order to answer the question correctly.
Grignard Reagents
Grignard reagents are organomagnesium compounds represented as R-MgX, where R is an organic group and X is a halogen. They are highly reactive and act as nucleophiles, allowing them to attack electrophilic carbon centers, such as those found in carbonyl compounds. This property makes them essential for forming new carbon-carbon bonds in organic synthesis.
Recommended video:
Carbonation of Grignard Reagents
Aldehyde Reactivity
Aldehydes are carbonyl compounds characterized by the presence of a carbonyl group (C=O) bonded to at least one hydrogen atom. They are more reactive than ketones due to the presence of the hydrogen, which makes the carbonyl carbon more electrophilic. This reactivity allows Grignard reagents to effectively add to aldehydes, leading to the formation of secondary alcohols upon hydrolysis.
Recommended video:
Synthesis of Secondary Alcohols
Secondary alcohols can be synthesized by the nucleophilic addition of Grignard reagents to aldehydes. When a Grignard reagent reacts with an aldehyde, it forms a tetrahedral intermediate, which upon protonation yields a secondary alcohol. Understanding the mechanism of this reaction is crucial for designing synthetic pathways in organic chemistry.
Recommended video:
Forming alcohols through SN2 reactions.
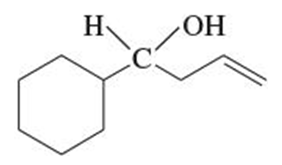
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 13:4m
13:4m