Here are the essential concepts you must grasp in order to answer the question correctly.
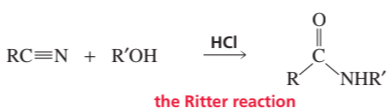
Ritter Reaction
The Ritter reaction is a chemical reaction that involves the conversion of a nitrile and an alcohol into an N-substituted amide in the presence of a strong acid, typically hydrochloric acid. This reaction is significant in organic synthesis as it allows for the formation of amides, which are important functional groups in various chemical compounds. The mechanism involves protonation of the alcohol, followed by nucleophilic attack by the nitrile.
Recommended video:
Nucleophilicity
Nucleophilicity refers to the ability of a nucleophile to donate an electron pair to an electrophile, forming a chemical bond. In the context of the Ritter reaction, the alcohol acts as a nucleophile. Primary alcohols are less nucleophilic compared to secondary or tertiary alcohols due to steric hindrance and the stability of the resulting carbocation, which affects their reactivity in this specific reaction.
Recommended video:
Steric Hindrance
Steric hindrance is the prevention of reactions at a particular location in a molecule due to the size of substituent groups. In the case of primary alcohols, the steric bulk around the hydroxyl group can hinder the approach of the nitrile, making it less favorable for the nucleophilic attack necessary for the Ritter reaction. This is why primary alcohols do not effectively participate in this reaction, unlike their secondary and tertiary counterparts.
Recommended video:
Understanding steric effects.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 9:32m
9:32m