Here are the essential concepts you must grasp in order to answer the question correctly.
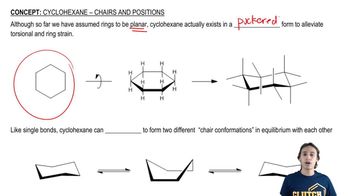
Chair Conformation of Cyclohexane
Cyclohexane can adopt a chair conformation, which minimizes steric strain and torsional strain. In this conformation, substituents can occupy either equatorial or axial positions. The equatorial position is generally more stable for larger substituents due to reduced steric hindrance with adjacent hydrogen atoms, making it crucial for understanding the stability of different substituents in cyclohexane.
Recommended video:
What is a chair conformation?
A Values
A values represent the free energy change (∆G°) associated with the transition of a substituent from the equatorial to the axial position in cyclohexane. Positive A values indicate that substituents favor the equatorial position due to lower energy and greater stability. These values are essential for calculating the equilibrium constant (K_eq) for chair-chair interconversions, as they quantify the preference of substituents for their respective positions.
Recommended video:
Calculating Chair Equilibrium
Equilibrium Constant (K_eq)
The equilibrium constant (K_eq) quantifies the ratio of the concentrations of products to reactants at equilibrium for a reversible reaction. In the context of chair-chair interconversions, K_eq can be calculated using the relationship K_eq = e^(-∆G°/RT), where R is the gas constant and T is the temperature in Kelvin. Understanding K_eq is vital for predicting the stability of different conformations and the distribution of substituents in cyclohexane.
Recommended video:
The relationship between equilibrium constant and pKa.