Here are the essential concepts you must grasp in order to answer the question correctly.
SN2 Mechanism
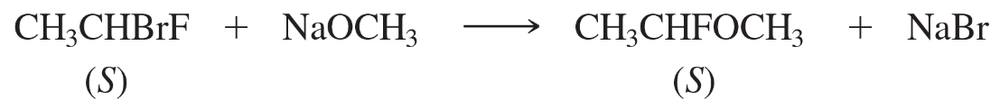
The SN2 mechanism is a type of nucleophilic substitution reaction where a nucleophile attacks an electrophile, resulting in the simultaneous displacement of a leaving group. This reaction occurs in a single concerted step, leading to inversion of configuration at the chiral center. The rate of the reaction depends on the concentration of both the nucleophile and the substrate, making it a bimolecular process.
Recommended video:
Drawing the SN2 Mechanism
Leaving Groups
In organic chemistry, a leaving group is an atom or group that can depart with a pair of electrons in a substitution or elimination reaction. The ability of a leaving group to stabilize the negative charge after departure is crucial; good leaving groups, like bromide, are typically weak bases. In this reaction, bromide is favored over fluoride due to its better leaving group ability, facilitating the SN2 process.
Recommended video:
The 3 important leaving groups to know.
Stereochemistry
Stereochemistry refers to the study of the spatial arrangement of atoms in molecules and how this affects their chemical behavior. In the context of this reaction, understanding whether the product retains or inverts configuration is essential. The stereochemical outcome is determined by the mechanism of the reaction; SN2 reactions typically result in inversion of configuration due to the backside attack of the nucleophile.
Recommended video:
Polymer Stereochemistry Concept 1
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 8:33m
8:33m