Here are the essential concepts you must grasp in order to answer the question correctly.
Electrophilic Aromatic Substitution
Electrophilic aromatic substitution (EAS) is a fundamental reaction in organic chemistry where an electrophile replaces a hydrogen atom on an aromatic ring, such as benzene. This process is crucial for synthesizing various derivatives of benzene, including p-nitro-N-methylaniline. Understanding the mechanism of EAS, including the role of catalysts and the stability of intermediates, is essential for predicting the outcomes of reactions involving aromatic compounds.
Recommended video:
Nitration of Aromatic Compounds
Nitration is a specific type of electrophilic aromatic substitution where a nitro group (NO2) is introduced into an aromatic ring. This reaction typically involves the use of a nitrating mixture, such as concentrated nitric acid and sulfuric acid, to generate the nitronium ion (NO2+), the active electrophile. Recognizing the conditions and regioselectivity of nitration is vital for synthesizing compounds like p-nitro-N-methylaniline from benzene.
Recommended video:
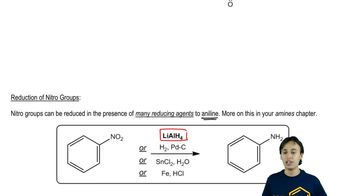
Reduction of Nitro Compounds
The reduction of nitro compounds is a key transformation in organic synthesis, where a nitro group is converted into an amine. This process can be achieved using various reducing agents, such as iron and hydrochloric acid or catalytic hydrogenation. Understanding the reduction mechanism is important for synthesizing p-nitro-N-methylaniline, as it involves the conversion of the nitro group to an amino group, which is essential for the final product.
Recommended video:
Reduction of Nitro Groups
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:49m
2:49m