Here are the essential concepts you must grasp in order to answer the question correctly.
Acyl Chlorides
Acyl chlorides, also known as acid chlorides, are reactive organic compounds derived from carboxylic acids by replacing the hydroxyl group (-OH) with a chlorine atom. They are commonly used in organic synthesis due to their ability to react with nucleophiles, such as amines, to form amides. Understanding the structure and reactivity of acyl chlorides is essential for determining the appropriate acyl chloride needed for amide synthesis.
Recommended video:
Recognizing acyl chlorides and anhydrides.
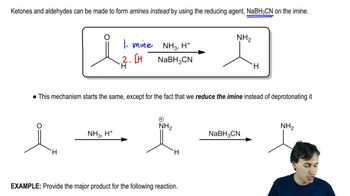
Amines
Amines are organic compounds that contain nitrogen atoms bonded to carbon atoms. They can be classified as primary, secondary, or tertiary based on the number of carbon groups attached to the nitrogen. In the context of amide synthesis, the type of amine used (e.g., primary or secondary) influences the structure of the resulting amide. Recognizing the differences between these amines is crucial for selecting the correct amine for the desired amide product.
Recommended video:
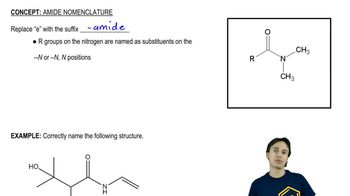
Amide Formation
Amide formation is a chemical reaction where an acyl chloride reacts with an amine to produce an amide and hydrochloric acid as a byproduct. This reaction is a key transformation in organic chemistry, often utilized in the synthesis of pharmaceuticals and other compounds. Understanding the mechanism of this reaction, including the role of nucleophilicity and electrophilicity, is vital for predicting the products formed in the synthesis of specific amides.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: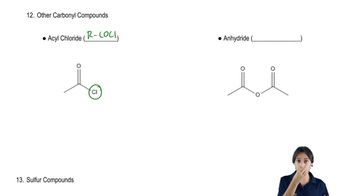

 9:32m
9:32m