Here are the essential concepts you must grasp in order to answer the question correctly.
Index of Hydrogen Deficiency (IHD)
The Index of Hydrogen Deficiency (IHD) is a measure of the degree of unsaturation in a molecular formula. It indicates the number of rings and/or multiple bonds present in a compound. Each double bond or ring contributes one to the IHD, while each triple bond counts as two. The formula for calculating IHD is IHD = (2C + 2 + N - H - X) / 2, where C is the number of carbons, N is the number of nitrogens, H is the number of hydrogens, and X is the number of halogens.
Recommended video:
What index of hydrogen deficiency is.
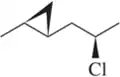
Chemical Structure Interpretation
Understanding chemical structures is crucial for determining the IHD. The structure provides information about the connectivity of atoms, the presence of functional groups, and the overall geometry of the molecule. In the provided image, the presence of a bromine atom and the cyclic structure indicates potential unsaturation, which must be accounted for when calculating the IHD.
Recommended video:
How to interpret condensed structures.
Halogens and Their Effect on IHD
Halogens, such as bromine, affect the calculation of the IHD because they can replace hydrogen atoms in a molecular formula. Each halogen atom is treated as if it were a hydrogen atom in the IHD formula. Therefore, when calculating IHD, the presence of bromine in the structure must be included in the count of atoms to ensure an accurate assessment of the compound's saturation level.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: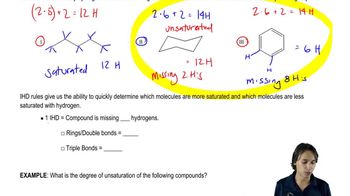


 2:39m
2:39m