Here are the essential concepts you must grasp in order to answer the question correctly.
1° Alcohols
Primary (1°) alcohols are organic compounds where the hydroxyl (-OH) group is attached to a carbon atom that is connected to only one other carbon atom. This structure influences their reactivity and properties, making them more susceptible to oxidation compared to secondary or tertiary alcohols.
Recommended video:
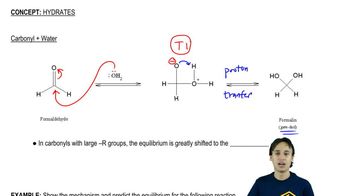
Formaldehyde as a Reactant
Formaldehyde (HCHO) is a simple aldehyde that can be used in organic synthesis to produce alcohols. In the presence of a suitable reducing agent, formaldehyde can react with Grignard reagents or organolithium compounds to yield primary alcohols, making it a key starting material in alcohol synthesis.
Recommended video:
Nucleophilic Addition Reactions
Nucleophilic addition reactions are fundamental in organic chemistry, where a nucleophile attacks an electrophilic carbon atom, leading to the formation of new bonds. In the context of synthesizing alcohols from aldehydes, the nucleophile (like a Grignard reagent) adds to the carbonyl carbon of formaldehyde, resulting in the formation of a primary alcohol after subsequent protonation.
Recommended video:

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 0:24m
0:24m