Here are the essential concepts you must grasp in order to answer the question correctly.
Nucleophilic Acyl Substitution
Nucleophilic acyl substitution is a fundamental reaction mechanism in organic chemistry where a nucleophile attacks the carbonyl carbon of a carboxylic acid derivative, leading to the substitution of a leaving group. This mechanism is crucial for understanding how different carboxylic acid derivatives, such as esters, amides, and acyl chlorides, react to form carbonyl products. The reaction typically involves the formation of a tetrahedral intermediate, which then collapses to release the leaving group.
Recommended video:
Nucleophiles and Electrophiles can react in Substitution Reactions.
Role of Acid Catalysts
Acid catalysts play a significant role in enhancing the reactivity of nucleophilic acyl substitution reactions. They can protonate the carbonyl oxygen, increasing the electrophilicity of the carbonyl carbon, making it more susceptible to nucleophilic attack. This is particularly important in reactions that may not proceed efficiently under neutral conditions, allowing for reactions that would otherwise be unfavorable to occur more readily.
Recommended video:
Acid-Base Catalysis Concept 3
Carboxylic Acid Derivatives
Carboxylic acid derivatives include compounds like acyl chlorides, esters, and amides, which differ in their leaving groups and reactivity. Understanding the structure and reactivity of these derivatives is essential for predicting the outcomes of nucleophilic acyl substitution reactions. Each type of derivative has unique characteristics that influence how they react with nucleophiles and the types of products formed, which is critical for answering questions about specific reaction pathways.
Recommended video:
Intro to Carboxylic Acid Derivatives
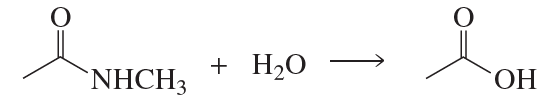
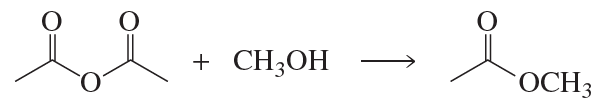
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 9:32m
9:32m