Here are the essential concepts you must grasp in order to answer the question correctly.
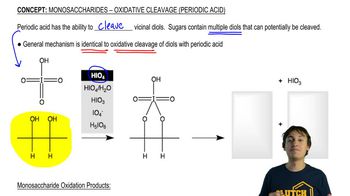
Periodic Acid Cleavage
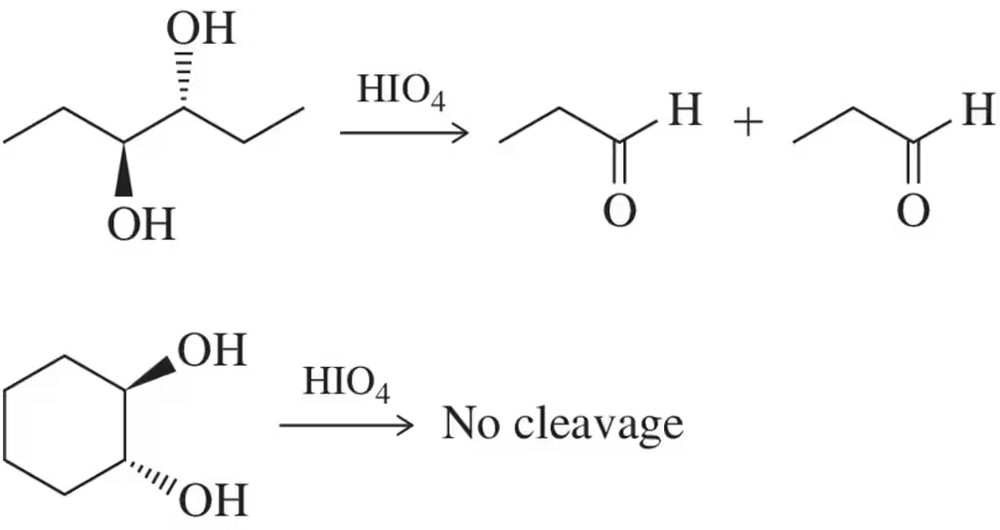
Periodic acid (HIO₄) is used to cleave vicinal diols, converting them into aldehydes or ketones. This reaction requires the diols to be in a specific orientation that allows the formation of a cyclic intermediate, which is crucial for the cleavage process. The ability to form this intermediate is influenced by the spatial arrangement of the diols.
Recommended video:
Monosaccharides - Oxidative Cleavage
Cyclic vs. Acyclic Diols
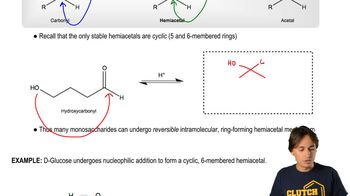
Cyclic diols are part of a ring structure, which restricts their spatial orientation due to the rigidity of the ring. This rigidity can prevent the necessary alignment for periodic acid cleavage. In contrast, acyclic diols have more freedom in their spatial arrangement, allowing them to adopt the conformation needed for the reaction to proceed.
Recommended video:
Monosaccharides - Forming Cyclic Hemiacetals
Stereochemistry of Diols
Stereochemistry refers to the spatial arrangement of atoms in molecules. For periodic acid cleavage, the stereochemistry of diols is crucial; trans-diols in rings often cannot achieve the required orientation due to steric hindrance and ring strain. Acyclic trans-diols, however, can rotate freely to align properly, facilitating the cleavage by periodic acid.
Recommended video:
Polymer Stereochemistry Concept 1
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: