Here are the essential concepts you must grasp in order to answer the question correctly.
Frost Circle
A Frost circle is a graphical representation used to visualize the energy levels of molecular orbitals in cyclic compounds. It helps in determining the stability and electronic configuration of species like cations and anions by plotting the energy levels against the vertices of a polygon, typically a circle. The position of the orbitals indicates their energy, allowing for easy comparison between different species.
Recommended video:
Cyclopentadienyl Cation and Anion
The cyclopentadienyl cation (C5H5+) and anion (C5H5-) are derived from cyclopentadiene by losing or gaining a hydrogen atom, respectively. The cation is electron-deficient and has a positive charge, while the anion is electron-rich and carries a negative charge. These changes in charge significantly affect their stability and reactivity, which can be analyzed through their respective Frost circles.
Recommended video:
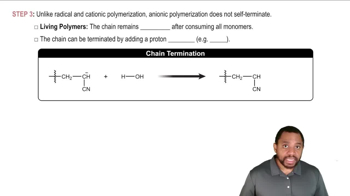
Anionic Polymerization Concept 4
Molecular Orbital Theory
Molecular Orbital Theory explains how atomic orbitals combine to form molecular orbitals, which can be occupied by electrons. In the context of the cyclopentadienyl cation and anion, this theory helps in understanding how the addition or removal of electrons alters the energy levels and distribution of electrons in the molecular orbitals, influencing the overall stability and properties of the species.
Recommended video:
Review of Molecular Orbitals

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 12:16m
12:16m