Here are the essential concepts you must grasp in order to answer the question correctly.
Oxidation Numbers
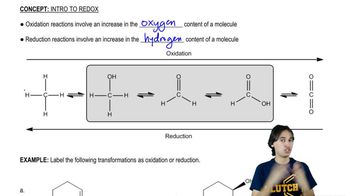
Oxidation numbers are a tool used in chemistry to keep track of electron transfer in chemical reactions. They are assigned to atoms based on a set of rules, reflecting the hypothetical charge an atom would have if all bonds were ionic. Understanding oxidation numbers is crucial for identifying changes in electron distribution, which is essential for determining redox reactions.
Recommended video:
Redox Reactions
Redox reactions, short for reduction-oxidation reactions, involve the transfer of electrons between two species. In these reactions, one substance is oxidized (loses electrons) and another is reduced (gains electrons). Identifying redox reactions requires analyzing changes in oxidation numbers, as these indicate electron transfer between reactants.
Recommended video:
General Features of Redox
Identifying Redox Reactions
To identify redox reactions, compare the oxidation numbers of elements in the reactants and products. A change in oxidation numbers indicates a redox process, where one element's oxidation number increases (oxidation) and another's decreases (reduction). This analysis helps determine if electron transfer has occurred, confirming the presence of a redox reaction.
Recommended video:
General Features of Redox

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 6:02m
6:02m