Here are the essential concepts you must grasp in order to answer the question correctly.
Acidity and pKa
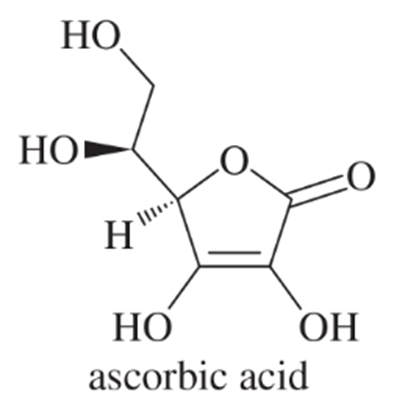
Acidity is a measure of a substance's ability to donate protons (H+ ions) in solution. The pKa value quantifies this acidity, with lower pKa values indicating stronger acids. Ascorbic acid has a pKa of 4.17, making it a stronger acid than acetic acid, which has a pKa of 4.74. Understanding these values helps compare the relative strengths of different acids.
Recommended video:
Conjugate Bases
When an acid donates a proton, it forms its conjugate base. The stability of the conjugate base significantly influences the acidity of the original acid. In the case of ascorbic acid, its conjugate base is stabilized by resonance and intramolecular hydrogen bonding, which can explain its comparable acidity to acetic acid, despite the absence of a carboxylic acid group.
Recommended video:
Resonance Stabilization
Resonance stabilization occurs when a molecule can be represented by multiple valid Lewis structures, allowing for the delocalization of electrons. This delocalization can enhance the stability of a conjugate base. In ascorbic acid, the presence of hydroxyl groups and the structure of the molecule allow for effective resonance stabilization, contributing to its acidity despite lacking a traditional carboxylic acid group.
Recommended video:
The radical stability trend.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 3:15m
3:15m