Here are the essential concepts you must grasp in order to answer the question correctly.
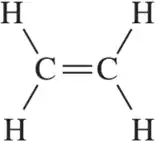
Lewis Structures
Lewis structures are diagrams that represent the bonding between atoms in a molecule and the lone pairs of electrons that may exist. They help visualize the arrangement of electrons and the connectivity of atoms, which is crucial for understanding molecular geometry and polarity.
Recommended video:
Drawing the Lewis Structure for N2H4.
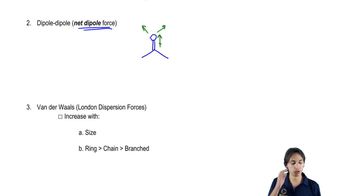
Dipole Moment
A dipole moment is a measure of the separation of positive and negative charges in a molecule, indicating its polarity. It is represented as a vector pointing from the positive to the negative charge, and its magnitude depends on the charge difference and the distance between the charges.
Recommended video:
How dipole-dipole forces work.
Molecular Polarity
Molecular polarity arises from the uneven distribution of electron density within a molecule, often due to differences in electronegativity between bonded atoms. A molecule is polar if it has a net dipole moment, which can affect its physical properties and interactions with other molecules.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: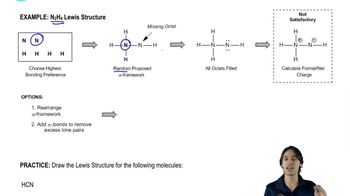


 11:33m
11:33m