Here are the essential concepts you must grasp in order to answer the question correctly.
Diels–Alder Reaction
The Diels–Alder reaction is a [4+2] cycloaddition reaction between a conjugated diene and a dienophile, resulting in the formation of a six-membered ring. This reaction is a powerful tool in organic synthesis due to its ability to create complex cyclic structures in a single step, often with high stereoselectivity and regioselectivity.
Recommended video:
Diels-Alder Retrosynthesis
Conjugated Dienes
Conjugated dienes are compounds that contain two double bonds separated by a single bond, allowing for resonance stabilization. This property enhances their reactivity in Diels–Alder reactions, as the electron-rich diene can effectively interact with an electron-deficient dienophile, facilitating the formation of new bonds.
Recommended video:
Stereochemistry in Diels–Alder Reactions
Stereochemistry plays a crucial role in Diels–Alder reactions, as the orientation of substituents on the diene and dienophile can influence the final product's configuration. The reaction typically leads to the formation of two new stereocenters, and understanding the stereochemical outcomes is essential for predicting the structure of the synthesized compounds.
Recommended video:
Diels-Alder Retrosynthesis
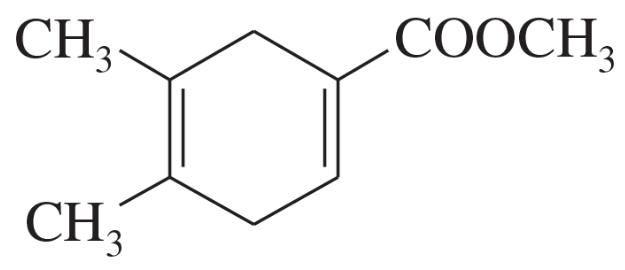
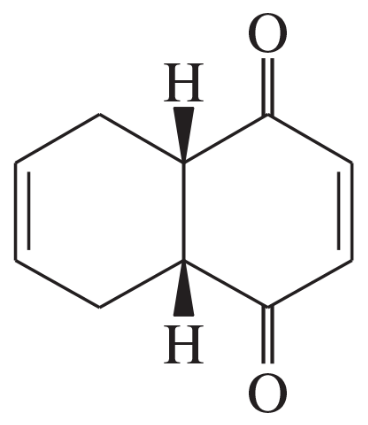
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 4:02m
4:02m