Here are the essential concepts you must grasp in order to answer the question correctly.
Markovnikov's Rule
Markovnikov's Rule states that when HX (where X is a halogen) adds to an alkene, the hydrogen atom will attach to the carbon with the greater number of hydrogen atoms already attached. This rule helps predict the major product of electrophilic addition reactions, particularly in the absence of peroxides.
Recommended video:
The 18 and 16 Electron Rule
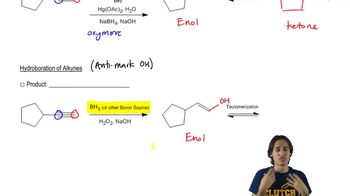
Anti-Markovnikov Addition
Anti-Markovnikov addition occurs when the addition of HX to an alkene results in the halogen attaching to the less substituted carbon. This is typically facilitated by the presence of peroxides, which lead to a radical mechanism that reverses the regioselectivity observed in standard electrophilic additions.
Recommended video:
Anti-Markovnikov addition of alcohols to terminal alkynes yields aldehydes
Radical Mechanism
A radical mechanism involves the formation of free radicals during a chemical reaction. In the context of alkene reactions with HBr in the presence of peroxides, this mechanism allows for the anti-Markovnikov addition, where the bromine atom attaches to the less substituted carbon, leading to different product outcomes compared to the non-radical pathway.
Recommended video:
The mechanism of Radical Polymerization.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 1:18m
1:18m