Here are the essential concepts you must grasp in order to answer the question correctly.
Saponification
Saponification is a chemical reaction that involves the hydrolysis of esters in the presence of a strong base, typically sodium hydroxide (NaOH) or potassium hydroxide (KOH). In this process, an ester reacts with hydroxide ions to produce an alcohol and a carboxylate salt. This reaction is commonly associated with the production of soap from fats and oils, where the ester bonds in triglycerides are broken down.
Recommended video:
Mechanism of Nucleophilic Substitution
The mechanism of nucleophilic substitution is a fundamental concept in organic chemistry where a nucleophile attacks an electrophile, resulting in the replacement of a leaving group. In the case of saponification, the hydroxide ion acts as the nucleophile, attacking the carbonyl carbon of the ester. This leads to the formation of a tetrahedral intermediate, which subsequently collapses to release the alcohol and the carboxylate ion.
Recommended video:
Nucleophiles and Electrophiles can react in Substitution Reactions.
Phenyl Esters
Phenyl esters are organic compounds formed from the reaction of phenol and a carboxylic acid, where the hydroxyl group of phenol is replaced by an acyl group. They are characterized by their aromatic ring and can undergo hydrolysis reactions, such as saponification. Understanding the structure and reactivity of phenyl esters is crucial for predicting the products of their reactions, particularly in the context of nucleophilic attack by hydroxide ions.
Recommended video:
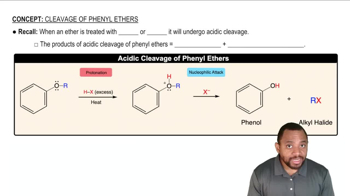
Cleavage of Phenyl Ethers Concept 1