Here are the essential concepts you must grasp in order to answer the question correctly.
Resonance Structures
Resonance structures are different ways of drawing the same molecule that illustrate the delocalization of electrons. They are used to represent molecules where the electron distribution cannot be depicted by a single Lewis structure. Each resonance structure contributes to the overall hybrid structure, which is more stable than any individual form.
Recommended video:
Drawing Resonance Structures
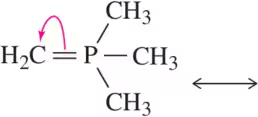
Electron Flow
Electron flow refers to the movement of electrons during chemical reactions or within molecules, often depicted using arrows in reaction mechanisms. Understanding electron flow is crucial for predicting how molecules will react and how resonance structures are formed, as it shows how electrons are redistributed among atoms.
Recommended video:
The 18 and 16 Electron Rule
Stability of Resonance Structures
The stability of resonance structures is determined by factors such as the octet rule, charge distribution, and the presence of formal charges. More stable resonance structures contribute more to the resonance hybrid, while less stable forms are less significant. Recognizing which structures are more stable helps in predicting the behavior of the molecule in chemical reactions.
Recommended video:
Drawing Resonance Structures
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 3:34m
3:34m