Here are the essential concepts you must grasp in order to answer the question correctly.
Equilibrium Constant (Keq)
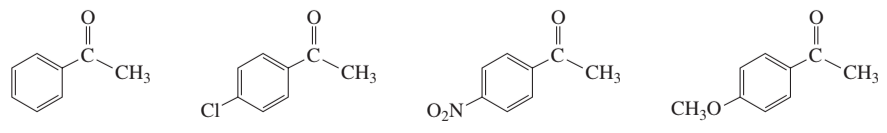
The equilibrium constant (Keq) quantifies the ratio of the concentrations of products to reactants at equilibrium for a reversible reaction. A larger Keq indicates a greater tendency for the formation of products, while a smaller Keq suggests that reactants are favored. In the context of hydrate formation, Keq helps determine which compound is more likely to form hydrates based on its structural features.
Recommended video:
The relationship between equilibrium constant and pKa.
Hydration and Hydrate Formation
Hydration refers to the process where water molecules interact with solute molecules, often leading to the formation of hydrates. The stability of a hydrate is influenced by the ability of the solute to form favorable interactions with water, such as hydrogen bonding. Compounds with functional groups that can engage in strong hydrogen bonding with water typically exhibit higher Keq values for hydrate formation.
Recommended video:
Influence of Functional Groups
Functional groups significantly affect the chemical properties and reactivity of organic compounds. In the context of hydrate formation, groups such as hydroxyl (-OH) and sulfonic acid (-SO3H) can enhance solubility and promote hydration due to their ability to form hydrogen bonds with water. The presence and type of functional groups in the compounds A, B, C, and D will influence their respective Keq values for hydrate formation.
Recommended video:
Identifying Functional Groups
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

