Here are the essential concepts you must grasp in order to answer the question correctly.
Williamson Ether Synthesis
The Williamson ether synthesis is a method for creating ethers through the nucleophilic substitution of an alkoxide ion with a primary alkyl halide. This reaction typically involves the deprotonation of an alcohol to form an alkoxide, which then acts as a nucleophile to attack an electrophilic carbon in an alkyl halide, resulting in ether formation. The choice of reactants and conditions is crucial for the success of this synthesis.
Recommended video:
The Mechanism of Williamson Ether Synthesis.
Protonation of Alcohols
Protonation of alcohols involves the addition of a proton (H+) to the hydroxyl group (-OH), converting it into a better leaving group, typically water (H2O). This process is essential in reactions where the alcohol is transformed into a more reactive species, facilitating nucleophilic substitution. However, protonation can lead to carbocation formation, which may result in rearrangements or elimination reactions instead of the desired substitution.
Recommended video:
Nucleophilic Substitution Mechanisms
Nucleophilic substitution mechanisms, such as SN1 and SN2, describe how nucleophiles replace leaving groups in organic reactions. In SN2 reactions, a strong nucleophile attacks the electrophilic carbon simultaneously as the leaving group departs, leading to a concerted mechanism. In contrast, SN1 involves the formation of a carbocation intermediate, followed by nucleophilic attack. Understanding these mechanisms is vital for predicting the outcomes of reactions involving ethers and alcohols.
Recommended video:
Nucleophiles and Electrophiles can react in Substitution Reactions.
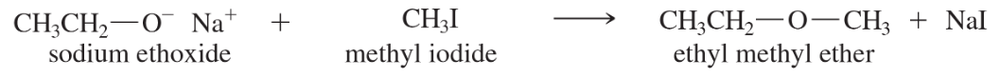
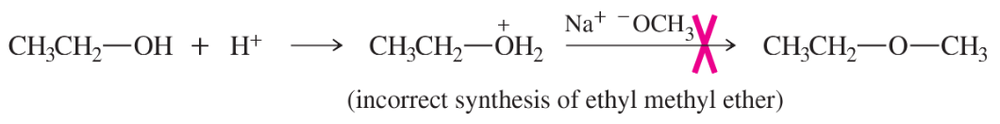
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 3:50m
3:50m