Textbook Question
Which of the following compounds are meso?
(a)
969
views

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: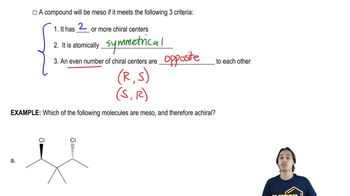



 3:52m
3:52mMaster Three types of disubstituted cycloalkanes with a bite sized video explanation from Johnny
Start learning