Here are the essential concepts you must grasp in order to answer the question correctly.
Protecting Groups
Protecting groups are temporary modifications used in organic synthesis to prevent certain functional groups from reacting during a chemical reaction. They allow for selective reactions to occur on other parts of the molecule without interference. After the desired reactions are completed, the protecting group can be removed to restore the original functional group.
Recommended video:
Nucleophilic Substitution Reactions
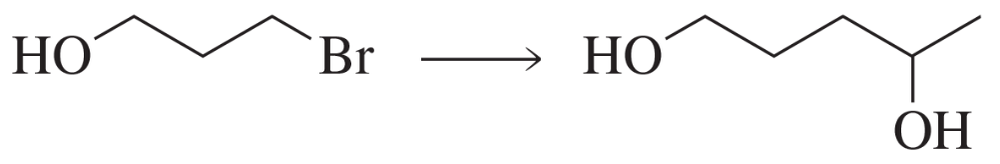
Nucleophilic substitution reactions involve the replacement of a leaving group (like bromine) by a nucleophile (such as an alcohol or an alkoxide). This type of reaction is fundamental in organic chemistry for forming new bonds and modifying molecular structures. The mechanism can proceed via either an SN1 or SN2 pathway, depending on the substrate and conditions.
Recommended video:
Nucleophiles and Electrophiles can react in Substitution Reactions.
Stereochemistry
Stereochemistry is the study of the spatial arrangement of atoms in molecules and how this affects their chemical behavior. In the context of organic reactions, understanding stereochemistry is crucial for predicting the outcomes of reactions, especially when chiral centers are involved. The configuration of the product can influence its reactivity and interactions in biological systems.
Recommended video:
Polymer Stereochemistry Concept 1
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 7:16m
7:16m