Here are the essential concepts you must grasp in order to answer the question correctly.
Nucleophilic Substitution Reactions
Nucleophilic substitution reactions involve the replacement of a leaving group (like Cl) by a nucleophile (such as H2O). In these reactions, the nucleophile attacks the electrophilic carbon atom bonded to the leaving group, leading to the formation of a new bond and the departure of the leaving group. Understanding the mechanism, whether it follows an SN1 or SN2 pathway, is crucial for predicting the product.
Recommended video:
Nucleophiles and Electrophiles can react in Substitution Reactions.
Carbocation Stability
In reactions involving carbocations, the stability of the carbocation intermediate significantly influences the reaction pathway. Tertiary carbocations are more stable than secondary or primary ones due to hyperconjugation and inductive effects. If a carbocation forms during the reaction, it may lead to rearrangements to form a more stable carbocation, which can affect the final product.
Recommended video:
Determining Carbocation Stability
Rearrangement in Substitution Reactions
Rearrangement in substitution reactions occurs when a carbocation intermediate can shift to a more stable configuration. This can involve hydride or alkyl shifts, allowing the molecule to achieve a lower energy state. Recognizing when and how rearrangements happen is essential for accurately predicting the final product of the reaction, especially in complex cyclic structures.
Recommended video:
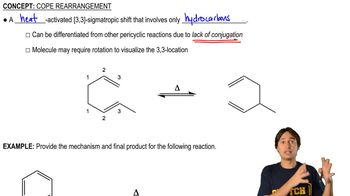
Definition of Cope Rearrangement

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 3:32m
3:32m