Here are the essential concepts you must grasp in order to answer the question correctly.
Protic Solvents
Protic solvents are those that have a hydrogen atom attached to an electronegative atom, typically oxygen or nitrogen, allowing them to form hydrogen bonds. These solvents can donate protons (H+) in chemical reactions, which is a key characteristic. Common examples include water, alcohols, and carboxylic acids.
Recommended video:
The difference between protic vs. aprotic solvents.
Aprotic Solvents
Aprotic solvents lack hydrogen atoms bonded to electronegative atoms, meaning they cannot donate protons. They can still accept protons, but they do not participate in hydrogen bonding in the same way as protic solvents. Examples include acetone, dimethyl sulfoxide (DMSO), and chloroform.
Recommended video:
The difference between protic vs. aprotic solvents.
Solvent Polarity
The polarity of a solvent affects its ability to dissolve various solutes and participate in chemical reactions. Protic solvents are generally polar due to their ability to form hydrogen bonds, while aprotic solvents can be polar or nonpolar. Understanding solvent polarity is crucial for predicting solubility and reactivity in organic chemistry.
Recommended video:
Identification of polarity in solvents
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution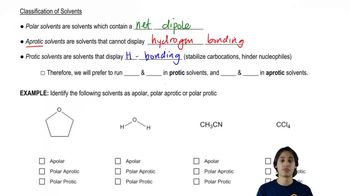



 2:26m
2:26m