Here are the essential concepts you must grasp in order to answer the question correctly.
Diels–Alder Reaction
The Diels–Alder reaction is a [4+2] cycloaddition reaction between a conjugated diene and a dienophile, resulting in the formation of a six-membered ring. This reaction is significant in organic synthesis due to its ability to create complex cyclic structures efficiently. Understanding the mechanism, including the roles of orbital overlap and electron density, is crucial for predicting the products and their stereochemistry.
Recommended video:
Diels-Alder Retrosynthesis
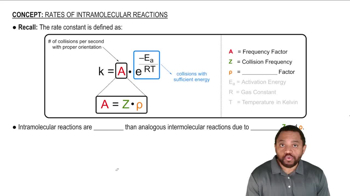
Intramolecular Reactions
Intramolecular reactions occur when the reactants are part of the same molecule, allowing for a more favorable reaction environment. In the context of the Diels–Alder reaction, this means that the diene and dienophile are connected, leading to the formation of multiple rings in a single step. This concept is essential for visualizing the spatial arrangement of atoms and predicting the resulting stereochemistry.
Recommended video:
Rates of Intramolecular Reactions Concept 1
Stereochemistry
Stereochemistry refers to the study of the spatial arrangement of atoms in molecules and how this affects their chemical behavior. In the Diels–Alder reaction, the stereochemical outcome is influenced by the orientation of the diene and dienophile during the reaction. Understanding stereochemistry is vital for predicting the configuration of the product, especially in cases where chiral centers are formed.
Recommended video:
Polymer Stereochemistry Concept 1
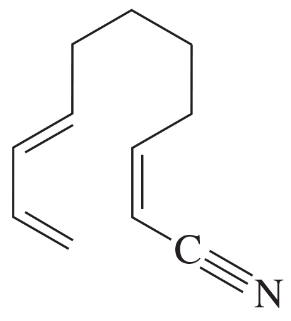
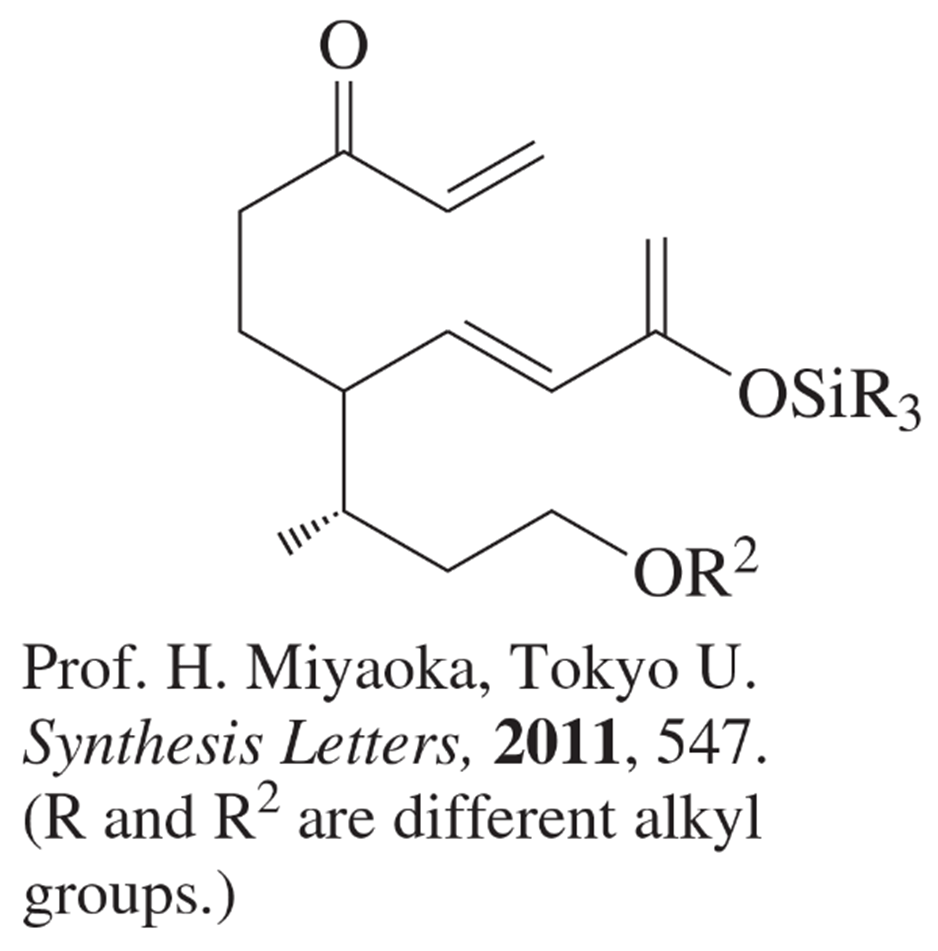
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: