Here are the essential concepts you must grasp in order to answer the question correctly.
Acyl Chlorides
Acyl chlorides, such as benzoyl chloride, are reactive compounds containing a carbonyl group (C=O) bonded to a chlorine atom. They are known for their ability to undergo nucleophilic acyl substitution reactions, where the chlorine atom is replaced by a nucleophile. Understanding the reactivity of acyl chlorides is crucial for predicting the products formed when they react with various nucleophiles.
Recommended video:
Recognizing acyl chlorides and anhydrides.
Nucleophilic Substitution
Nucleophilic substitution is a fundamental reaction mechanism in organic chemistry where a nucleophile attacks an electrophilic carbon atom, leading to the replacement of a leaving group. In the case of benzoyl chloride, nucleophiles like benzylamine and 4-chlorophenol can attack the carbonyl carbon, resulting in the formation of amides or esters, respectively. This concept is essential for understanding how different reagents interact with acyl chlorides.
Recommended video:
Nucleophiles and Electrophiles can react in Substitution Reactions.
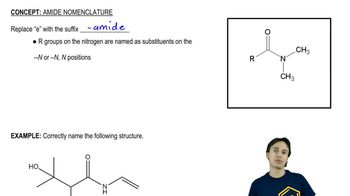
Amide Formation
Amide formation occurs when an acyl chloride reacts with an amine, resulting in the creation of an amide bond (C=O-N). In the reaction with excess benzylamine, benzoyl chloride will yield N-benzoylbenzylamine, showcasing the transformation of the acyl chloride into a more stable amide. This process is significant in organic synthesis, as amides are important functional groups in various chemical compounds.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: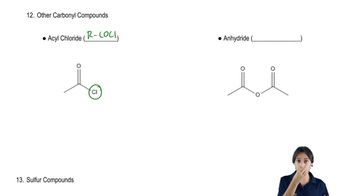


 9:32m
9:32m