Here are the essential concepts you must grasp in order to answer the question correctly.
Enzyme Inhibition
Enzyme inhibition refers to the process by which a molecule (inhibitor) decreases the activity of an enzyme. Inhibitors can be competitive, non-competitive, or uncompetitive, depending on how they interact with the enzyme and its substrate. Understanding the type of inhibition is crucial for proposing a mechanism, as it influences how the inhibitor affects the enzyme's active site and overall function.
Recommended video:
Intro to Coenzymes Example 1
Penicillinase Mechanism
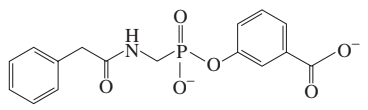
Penicillinase, also known as beta-lactamase, is an enzyme that hydrolyzes the beta-lactam ring of penicillin, rendering it ineffective. The mechanism of penicillinase involves the formation of an acyl-enzyme intermediate, where the enzyme covalently binds to the antibiotic. Recognizing this mechanism is essential for understanding how inhibitors can prevent the formation of this intermediate and restore the antibiotic's efficacy.
Recommended video:
Hydroxylamine Reactivation
Hydroxylamine (NH2OH) is a nucleophilic reagent that can reactivate certain enzymes by cleaving acyl-enzyme intermediates formed during inhibition. In the context of penicillinase, hydroxylamine can attack the acyl-enzyme complex, leading to the release of the enzyme and restoring its activity. This reactivation process is important for understanding the dynamics of enzyme inhibition and the potential for reversing the effects of inhibitors.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 5:53m
5:53m