Here are the essential concepts you must grasp in order to answer the question correctly.
Fischer Esterification
Fischer esterification is a chemical reaction that forms an ester from a carboxylic acid and an alcohol in the presence of an acid catalyst. The reaction involves the protonation of the carbonyl oxygen, making the carbon more electrophilic, which facilitates the nucleophilic attack by the alcohol. This process is reversible and typically requires heat to drive the reaction towards ester formation.
Recommended video:
Reactions of Amino Acids: Esterification Concept 1
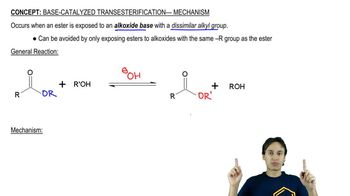
Transesterification
Transesterification is the process of exchanging the alkoxy group of an ester with an alcohol, resulting in a new ester and a new alcohol. This reaction can be catalyzed by either acids or bases, but the base-catalyzed mechanism involves the deprotonation of the alcohol, which enhances its nucleophilicity. The similarity in mechanisms between transesterification and Fischer esterification highlights the role of the catalyst in facilitating the reaction.
Recommended video:
Base-Catalyzed Mechanism Limitations
Fischer esterification cannot be effectively catalyzed by a base because the reaction requires protonation of the carbonyl group to increase its electrophilicity. A base would deprotonate the alcohol instead, preventing the formation of the necessary protonated intermediate. This lack of protonation inhibits the nucleophilic attack on the carbonyl carbon, rendering the base-catalyzed Fischer esterification ineffective.
Recommended video:
 Verified step by step guidance
Verified step by step guidance Verified Solution
Verified Solution

 2:49m
2:49m